��Ŀ����
NO��������Ⱦ��������������������������������NO����������������֯�д��ڣ���������Ѫ�ܡ���ǿ��������Ĺ��ܣ������Ϊ������ѧ���о��ȵ㣬NO�౻��Ϊ�����Ƿ��ӡ�����1��NO��Դ��
A���������ɻ�β�� B����ҵ��������β�� C����������
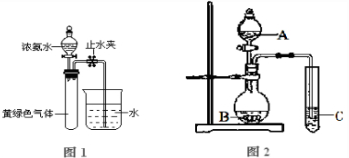
��2��д��ʵ������ȡNO�Ļ�ѧ����ʽ��
��3����ҵ�Ͽɲ��ü�Һ���պͰ���ԭ������NO��β���������ü�Һ���յĻ�ѧ����ʽΪ��NO+NO2+2NaOH�T2NaNO2+H2O��NO2+2NaOH�TNaNO2+NaNO3+H2O����������ԭ�������������б�����NaOH��Һ���պ�������ʣ�����
A��1mol O2 ��4mol NO2 B��1mol NO ��4mol NO2
C��1mol O2 ��7mol NO D��4mol NO ��4mol NO2��
��������1�����ݿ��Բ���һ��������;�����ش�
��2��ʵ�����ý���ͭ��ϡ����֮��ķ�Ӧ����ȡNO��
��3����������������ʽ��NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+2NaOH�TNaNO2+NaNO3+H2O��֪NO��NO2�������Ϊ1��1���ñ����գ��ٸ��ݵڶ�������ʽ��֪����������ֱ�ӱ�����������Һ���գ��ٽ��о��������
��2��ʵ�����ý���ͭ��ϡ����֮��ķ�Ӧ����ȡNO��
��3����������������ʽ��NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+2NaOH�TNaNO2+NaNO3+H2O��֪NO��NO2�������Ϊ1��1���ñ����գ��ٸ��ݵڶ�������ʽ��֪����������ֱ�ӱ�����������Һ���գ��ٽ��о��������
����⣺��1��A���ִ����п�����Ⱦ��֮һNO��Ҫ��Դ������β������A��ȷ��
B����ҵ�����ᣬβ���к���NO����B��ȷ��
C�����������������£����������������ΪNO����C��ȷ��
��ѡABC��
��2��ʵ�����ý���ͭ��ϡ����֮��ķ�Ӧ����ȡNO��ԭ������ʽΪ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2���ʴ�Ϊ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2��
��3��A��1mol O2 ��4mol NO2��ǡ����ȫת��Ϊ���ᣬ�ܱ������������գ���A����
B�����ݷ�Ӧ��NO+NO2+2NaOH�T2NaNO2+H2O��NO��������ϣ���������ʣ�࣬���Ƕ���������ֱ�ӱ�����������Һ���գ����ܱ������������գ���B����
C��1molO2��7molNO��Ϻ�Ӧ���ɶ���������ͬʱ����NOʣ�࣬ʣ���NOû��������������ȫ���գ���C��ȷ��
D��4mol NO��4mol NO2���������1��1�����ݷ�ӦNO+NO2+2NaOH�T2NaNO2+H2O֪�����ܱ���ȫ���գ���D����
ѡ��C��
B����ҵ�����ᣬβ���к���NO����B��ȷ��
C�����������������£����������������ΪNO����C��ȷ��
��ѡABC��
��2��ʵ�����ý���ͭ��ϡ����֮��ķ�Ӧ����ȡNO��ԭ������ʽΪ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2���ʴ�Ϊ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2��
��3��A��1mol O2 ��4mol NO2��ǡ����ȫת��Ϊ���ᣬ�ܱ������������գ���A����
B�����ݷ�Ӧ��NO+NO2+2NaOH�T2NaNO2+H2O��NO��������ϣ���������ʣ�࣬���Ƕ���������ֱ�ӱ�����������Һ���գ����ܱ������������գ���B����
C��1molO2��7molNO��Ϻ�Ӧ���ɶ���������ͬʱ����NOʣ�࣬ʣ���NOû��������������ȫ���գ���C��ȷ��
D��4mol NO��4mol NO2���������1��1�����ݷ�ӦNO+NO2+2NaOH�T2NaNO2+H2O֪�����ܱ���ȫ���գ���D����
ѡ��C��
���������⿼��ѧ������������IJ����Լ�����֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǽ���Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
�����Ŀ