��Ŀ����
2008�걱�����˻����û��ȼ��Ϊ���飨C3H8����Ϥ����˻����û��ȼ��Ϊ65%���飨C4H10����35%���飨C3H8������֪������1mol����ȼ�շų�2220kJ������1mol������ȼ�շų�2878kJ������1mol�춡��ȼ�շų�2869.6kJ�������Իش��������⣺
��1����ʾ������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ________��
��2�������й�˵����ȷ����________��
A.���˻��ȼ��ʱ������ת����Ҫ���ɻ�ѧ��ת��Ϊ����
B.��ͬ�����£����������ֵ�ȱ����
C.��������춡�鲻�ȶ�
D.�춡������е�̼�����������Ķ�
��3����֪1mol H2ȼ������Һ̬ˮ�ų�������285.8 kJ������5mol �����ͱ���Ļ�����壬��ȫȼ��ʱ����3847kJ���������ͱ���������Ϊ________��
C4H10(�����飬g) + 13/2O2(g)��4CO2(g)+5H2O(l) ��H=" -2878" kJ?mol-1_ A C 3:1
��2��B��������������֪��λ�����ı�����������ȼ�շų��������ֱ�Ϊ50.5kJ��49.6��C�����������������춡��ȼ�շų�������ǰ�ߴ���������������������Բ��ȶ���D�����ߵ�̼�����Ŀ��ͬ��
��3��������Ϊxmol����285.8x �� 2220����5��x��=3847����⼴��
��2��B��������������֪��λ�����ı�����������ȼ�շų��������ֱ�Ϊ50.5kJ��49.6��C�����������������춡��ȼ�շų�������ǰ�ߴ���������������������Բ��ȶ���D�����ߵ�̼�����Ŀ��ͬ��
��3��������Ϊxmol����285.8x �� 2220����5��x��=3847����⼴��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
2008 �걱�����˻��������������κ��ơ����� The Bird Nest ������һ������Ҳ�������ʳ�Ϊ��ʽ���飮��ʽ����� B10H14����ˮ��Ӧ�⣬���������ˮ��Ӧ�������������ᣬ�����ױ���������ͼ�����ֳ�ʽ���飬�й�˵����ȷ���ǣ�������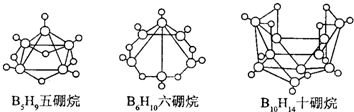

| A�����ೲʽ�����ͨʽ�� CnHn+4 | ||||
B��2B5H9+12O2
| ||||
| C��8 ����ԭ�ӵij�ʽ���黯ѧʽӦΪ B8H10 | ||||
| D��������ˮ��Ӧ�Ƿ�������ԭ��Ӧ |
2008�걱�����˻���������--����������̩��ʿ������Ϊȫ����ǿ�������̣����������˸�ǿ�ȡ������ܵķ����Ͻ���¸ֺ�884��ETFEĤ����������һ���ĵ����ء���������Щ������LED���ϣ��й�˵����ȷ���ǣ�������
| A���Ͻ���۵�ͨ������ֽ����ߣ�Ӳ�ȱ���ֽ���С | B����֪Ga���ڢ�A�壬����֪�����ػ�ѧʽΪGa3N2 | C���ý�������V2O5ұ���������������� | D��ETFEĤ��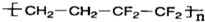 ���������ֵ���Ӿ۶��ɵ� ���������ֵ���Ӿ۶��ɵ� |
Ϊ��2008�걱�����˻��������ġ���ɫ���ˡ�����������������ŷţ�����Ϊ������Դ����������Ϊ���н�ͨȼ�ϵ��ǣ�������
| A������ | B���Ҵ� | C������ | D����Ȼ�� |