��Ŀ����
ij�ֶԱ����������������������ܼ�����ṹ��ʽ��ͼ��ʾ����R��ʾ��ͬ����������
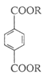
I����ͬѧͨ��ȼ�շ�ȷ��ij�Ա���������M�ĺ�����������������Ϊ23%����ش��������⣺
(1) ��ȷ��M�ķ���ʽΪ______________��
(2) �йضԱ���������M��˵����ȷ����______________��
II����ͬѧģ�ҵ������AΪԭ�ϺϳɶԱ���������M��ԭ������������̣�
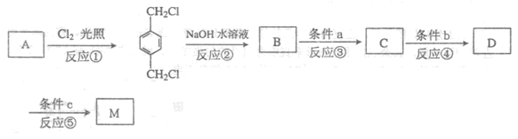
��ش��������⣺
(1) ��A�ж���ͬ���칹��,���к��б�����ֻ��һ��֧����ͬ���칹���������________��
(2) ע����Ӧ���ͣ���Ӧ��______ _����Ӧ��____ ___��
(3) д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��__________________________________________��
��Ӧ��__________________________________________

I����ͬѧͨ��ȼ�շ�ȷ��ij�Ա���������M�ĺ�����������������Ϊ23%����ش��������⣺
(1) ��ȷ��M�ķ���ʽΪ______________��
(2) �йضԱ���������M��˵����ȷ����______________��
| A��M����ϡ������Һ��Ӧ���ɴ������� | |
| B��Imol M�ڴ��������������������5mol H2��Ӧ | |
| C��1mol M����2mol NaOH����ˮ�ⷴӦ | D��M�� ��Ϊͬϵ�� ��Ϊͬϵ�� |

��ش��������⣺
(1) ��A�ж���ͬ���칹��,���к��б�����ֻ��һ��֧����ͬ���칹���������________��
(2) ע����Ӧ���ͣ���Ӧ��______ _����Ӧ��____ ___��
(3) д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��__________________________________________��
��Ӧ��__________________________________________
(1) C1 6H 2 2O4
(2) AC
(1)�ұ���
(2)��ȡ�� ��������
(3)��ѧ����ʽ��
(2) AC
(1)�ұ���
(2)��ȡ�� ��������
(3)��ѧ����ʽ��
I����1��M��Է�������===== ����M(C8H4O4R2)=278����R=57���ɴ˿ɵá�RΪ��C4H9������M�ķ���ʽΪC1 6H 2 2O4��
����M(C8H4O4R2)=278����R=57���ɴ˿ɵá�RΪ��C4H9������M�ķ���ʽΪC1 6H 2 2O4��
��2�������������Ľṹ��ʽΪ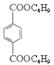 ����֪A��C��ȷ��
����֪A��C��ȷ��
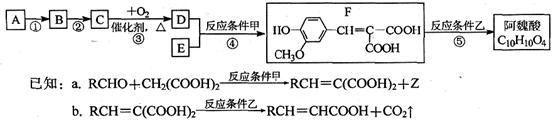
II���������̵ã�AΪ ��BΪ
��BΪ ��CΪ
��CΪ ��DΪ
��DΪ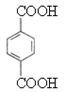
��1��AΪ�Զ��ױ�����ͬ���칹���к��б�����ֻ��һ��֧����ͬ���칹��Ϊ���ұ���
��2����Ӧ��Ϊȡ����Ӧ����Ӧ��Ϊ������Ӧ��
��3����Ӧ�ڷ���ʽΪ��

��Ӧ�ܷ���ʽΪ��
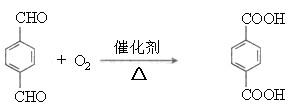
 ����M(C8H4O4R2)=278����R=57���ɴ˿ɵá�RΪ��C4H9������M�ķ���ʽΪC1 6H 2 2O4��
����M(C8H4O4R2)=278����R=57���ɴ˿ɵá�RΪ��C4H9������M�ķ���ʽΪC1 6H 2 2O4����2�������������Ľṹ��ʽΪ
 ����֪A��C��ȷ��
����֪A��C��ȷ��II���������̵ã�AΪ
 ��BΪ
��BΪ ��CΪ
��CΪ ��DΪ
��DΪ
��1��AΪ�Զ��ױ�����ͬ���칹���к��б�����ֻ��һ��֧����ͬ���칹��Ϊ���ұ���
��2����Ӧ��Ϊȡ����Ӧ����Ӧ��Ϊ������Ӧ��
��3����Ӧ�ڷ���ʽΪ��

��Ӧ�ܷ���ʽΪ��


��ϰ��ϵ�д�
�����Ŀ

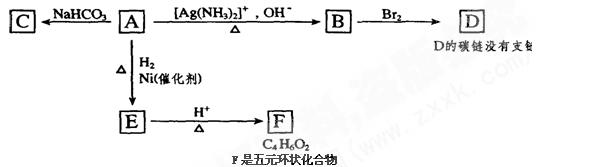
 ��д��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________________��
��д��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________________��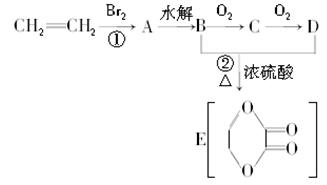
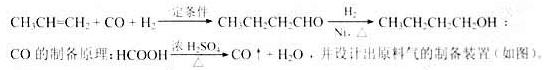
 ,
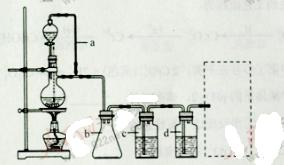
,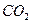 ��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳����________________������ţ�
��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳����________________������ţ�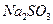 ��Һ ������
��Һ ������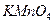 ��Һ ��ʯ��ˮ
��Һ ��ʯ��ˮ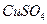 ��Ʒ����Һ
��Ʒ����Һ �����ͣ���
�����ͣ���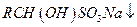 ���ڷе㣺����34��C��1-����118��C������Ƴ������ᴿ·�ߣ�
���ڷе㣺����34��C��1-����118��C������Ƴ������ᴿ·�ߣ�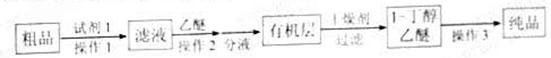
 ��ͼ��
��ͼ��


 ��
�� ʵ�飬��ʵ������漰�ķ�Ӧ������ ����������Ӧ���Ⱥ�˳����д����
ʵ�飬��ʵ������漰�ķ�Ӧ������ ����������Ӧ���Ⱥ�˳����д����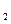 �������ʷ������Ҹ�����̼ԭ�ӣ����ͬ���칹������ �ֹ����š�
�������ʷ������Ҹ�����̼ԭ�ӣ����ͬ���칹������ �ֹ����š�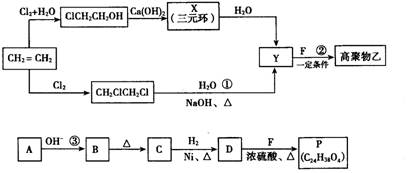
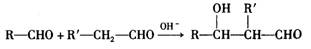 ��R��R��������������ԭ�ӣ���
��R��R��������������ԭ�ӣ���