��Ŀ����
��1����5�֣�
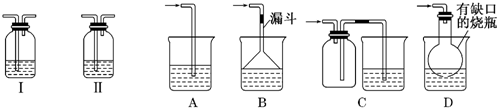
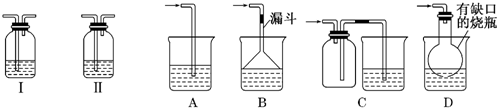
��ͼ�е�װ�ÿ�������ȡ��Ȳ����ش�ͼ��A�ܵ�����_______________
��ȡ��Ȳ�ķ���ʽ�ǣ�___________________________��
Ϊ���ⷴӦ̫Ѹ�٣��ɲ�ȡ�Ĵ�ʩ��______________________��

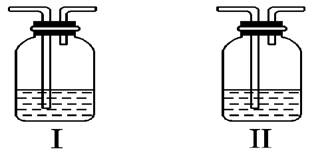
��2����7�֣�1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g・mL���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ��_________________________ �� __________________________ ��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е���__________________________
������c ��NaOH��Һ�������� ________________________
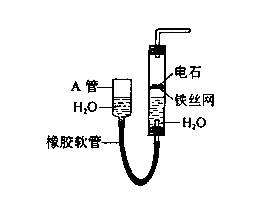
��1����5�֣�����ˮ��߶��Կ��Ʒ�Ӧ�ķ�����ֹͣ��2�֣���
CaC2+2H2O��Ca (OH)2+C2H2����2�֣� ����Ȳ��д�ṹ��ʽ��
�ñ���ʳ��ˮ����ˮ��1�֣�
��2����7�֣�CH3CH2OH �� CH2��CH2����H2O��2�֣���������Ũ���ᣬ���ȣ�CH2��CH2��Br2 �� CH2BrCH2Br��2�֣�
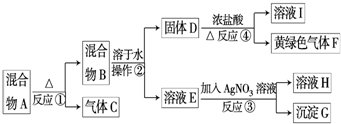
��3��b��ˮ���½�����������ˮ�����������������2�֣�
��4�� ��ȥ��ϩ�е��������壨SO2��CO2����1�֣�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�