��Ŀ����
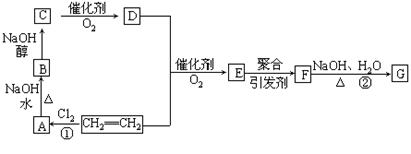
����ѧ���������������ز��������;��ʯ�Ͳ�Ʒ��ϩΪ��ʼԭ�Ͻ��кϳɸ߷��ӻ�����F��G���ϳ�·����ͼ��ʾ��
��֪��E�ķ���ʽΪC4H6O2��F�ķ���ʽΪ(C4H6O2)n���������齺������Ҫ�ɷ֣���G�ķ���ʽΪ(C2H4O)n���������ƻ�ѧ��������2CH2=CH2+2CH3COOH+O2  2C4H6O2��������ϩ����+2H2O
2C4H6O2��������ϩ����+2H2O
��֪����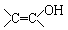 �ṹ���Ƶ��л��ﲻ�ȶ�������������������
�ṹ���Ƶ��л��ﲻ�ȶ�������������������
��ش��������⣺
��1��д���ṹ��ʽ��D_______________��F___________________��
��2����Ӧ�١��ڵķ�Ӧ����______________��___________________________��
��3��д�������йط�Ӧ�ĵĻ�ѧ����ʽ��
A����B________________________________________________
B����C _______________________________________________
��1��DΪCH3COOH��FΪ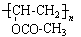 ����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
��3��ClCH2CH2Cl+H2O 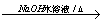 ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH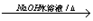 CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��
���������������ͼ�����ƶϳ�AΪClCH2CH2Cl��BΪClCH2CH2OH��CΪCH3CHO��DΪCH3COOH��FΪ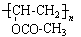 ���ԣ�1���У�DΪCH3COOH��FΪ
���ԣ�1���У�DΪCH3COOH��FΪ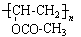 ����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2
����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2 ClCH2CH2Cl��
ClCH2CH2Cl��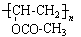
 (C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O
(C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O  ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��
���㣺�л��ƶ���
������������һ���л��ƶ��⣬�Ǹ߿�������ص㡣���и�������Ϣ���ϴ��������˵���DZȽ����ġ�

 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�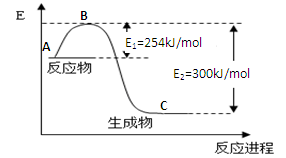
 2C4H6O2��������ϩ����+2H2O
2C4H6O2��������ϩ����+2H2O �ṹ���Ƶ��л��ﲻ�ȶ�������������������
�ṹ���Ƶ��л��ﲻ�ȶ�������������������
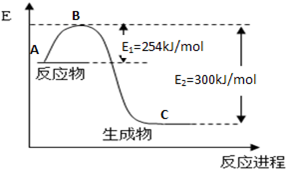
 N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���
N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���