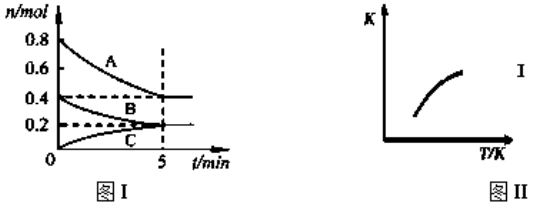
��Ŀ����
����Ŀ����1����0.2mol/L HA ��Һ�� 0.1mol/L NaOH��Һ�������ϣ���û����Һ��c��Na+��>c��A������������>������<������=����д���пհ�����
�������Һ��c��A��_________c��HA����
�������Һ��c��HA�� + c��A�� 0.1mol/L��
��2������ʱ��ȡ0.1molL-1 HX��Һ��0.1molL-1 NaOH��Һ������������Ϻ���Һ����ı仯��������û����Һ��pH=8���Իش��������⣺
�������Һ����ˮ�������c��OH-����0.1molL-1 NaOH��Һ����ˮ�������c��OH-��֮��Ϊ ��
����֪NH4X��Һ�����ԣ���֪��HX��Һ���뵽Na2CO3��Һ��������ų������ƶ���NH4��2CO3��Һ��pH__________7��ѡ����������������������������
���𰸡�
��1��������������
��2����107��1��107��������
��������
�����������1������0.2mol/L HA ��Һ�� 0.2mol/L NaOH��Һ�������ϣ�����Һ�е�������NaA�����ʵ���Ũ����0.1mol/L�������Һ��c��Na+����c��A-����˵��A-����ˮ���Լ��ԣ�A-+H2O![]() HA+OH-��c��HA����c��A-�����ʴ�Ϊ������
HA+OH-��c��HA����c��A-�����ʴ�Ϊ������
����Һ��c��H+����c��OH-������ҺNaA�ʼ��ԣ����������غ��c��HA��+c��A-��=0.1mol/L���ʴ�Ϊ��=��
��2������ʱ��ȡ0.1molL-1HX��Һ��0.1molL-1 NaOH��Һ������������Ϻ���Һ����ı仯���������������ʵ�����ȣ�����ǡ�÷�Ӧ����NaX����û����Һ��pH=8�������Һ�ʼ��ԣ�˵��NaX��ǿ�������Σ���HX�����
�������Һ����ˮ�������c��OH-��=![]() mol/L=10-6mol/L��0.1mol/LNaOH��Һ����ˮ�������c��OH-��=
mol/L=10-6mol/L��0.1mol/LNaOH��Һ����ˮ�������c��OH-��=![]() mol/L=10-13mol/L�������Һ����ˮ�������c��OH-����0.1molL-1NaOH��Һ����ˮ�������c��OH-��֮��=10-6mol/L��10-13mol/L=107��1���ʴ�Ϊ��107��1��
mol/L=10-13mol/L�������Һ����ˮ�������c��OH-����0.1molL-1NaOH��Һ����ˮ�������c��OH-��֮��=10-6mol/L��10-13mol/L=107��1���ʴ�Ϊ��107��1��
��NH4X��Һ�����ԣ�˵��NH4+��X-����ˮ��̶���ȣ���һˮ�ϰ���HX����ƽ�ⳣ����ȣ�HX��Һ���뵽Na2CO3��Һ��������ų���˵��HX���Դ���H2CO3����CO32-ˮ��̶ȴ���NH4+�����Ը���Һ�ʼ��ԣ�pH��7���ʴ�Ϊ������