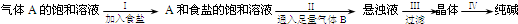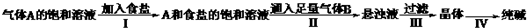��Ŀ����
�ҹ���ѧ�Һ�°����NaHCO3�ܽ�ȱ�NaCl��Na2CO3��NH4HCO3��NH4Cl��С�����ʣ�����CO2 +NH3 +H2O+NaCl��NaHCO3��+NH4Cl�ķ�Ӧԭ���Ʊ������������ʵ���ҽ���ģ��ʵ�����������ʾ��ͼ��
 |
�����������������
A��A������NH3��B������CO2
B���Ѵ���ڢõ��ľ�����ijЩ�����������ʣ����ʯ�ᣩ��Ͽ��Ƶ÷��ͷ�
C������ɹ㷺�����ڲ�������������ֽ����֯�ȹ�ҵ��
D���ڢ��������ǽ���������ˮ����ȡ��������ᾧ
D

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�ҹ���ѧ�Һ�°����NaHCO3�ܽ�ȱ�NaCl��Na2CO3��NH4HCO3��NH4Cl��С�����ʣ�����CO2+NH3+H2O+NaCl=NaHCO3��+NH4Cl�ķ�Ӧԭ���Ʊ������������ʵ���ҽ���ģ��ʵ�����������ʾ��ͼ��
����A�ı�����Һ
A��ʳ�εı�����Һ
����Һ
����
����
����������������ǣ�������
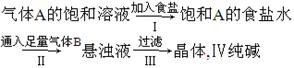
����A�ı�����Һ
| ����ʳ�� |
| �� |
| ͨ����������B |
| �� |
| ���� |
| �� |
| �� |
����������������ǣ�������
| A��A������CO2��B������NH3 |
| B���ڢõ��ľ����Ƿ��ͷ۵���Ҫ�ɷ� |
| C���ڢ����õ�����Ҫ�����������ձ���©���������� |
| D���ڢ�����������Ҫ�������ܽ⡢�������ᾧ |