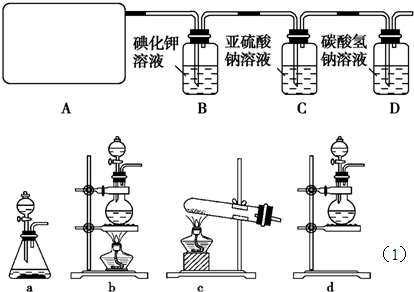
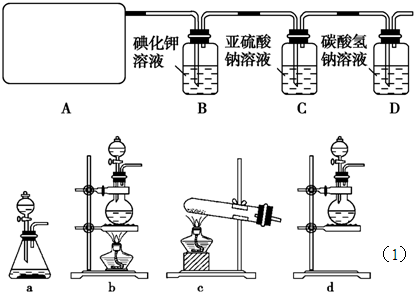
题目内容
(14分)某研究性学生小组查阅资料得知,漂白粉与硫酸溶液反应可制取氯气,化学方程式为:Ca(ClO)2+CaC l2+2H2SO4
l2+2H2SO4 2CaSO4+2Cl2↑+2H2O他们设计了如图所示装置制取氯气并验证其性质的实验。
2CaSO4+2Cl2↑+2H2O他们设计了如图所示装置制取氯气并验证其性质的实验。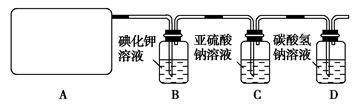
试回答:
(1)该实验中A部分的装置是________(填写装置的序号);
(2)B中反应的化学方程式是 。
(3)写出C中反应的离子方程式 ,并请你帮该小组同学设计一个实验,证明洗气瓶C中的Na2SO3已被氧化(简述实验步骤):_ ________________ 。
________________ 。
(4)写出在D装置中发生反应的离子方程式 。
(5)该实验存在明显的缺陷是__________________________________。
(6)该小组又进行了如下实验:称取漂白粉2.0 g,研磨后溶解,配制成250 mL溶液,加入过量的KI溶液和过量的H2SO4溶液,静置。待完全反应后,用0.1 mol·L-1的Na2S2O3溶液做标准溶液滴定反应生成的碘,已知反应式为2Na2S2O3+I2===Na2S4O6+2NaI,反应完成时,共消耗Na2S2O3 200 mL。则该漂白粉中Ca(ClO)2的质量分数为___ _____。
(每空2分,共14分)
(1)b (2)Cl2+2KI=2KCl+I2 (3)Cl2+SO32-+H2O=SO42-+2Cl-+2H+;取少量反应后的溶液于试管中,加入HCl溶液至不再产生气体为止,再滴加BaCl2溶液,如果有白色沉淀生成,证明Na2SO3已被氧化。
(4)Cl2+H2O H++Cl-+HClO ,HCO3-+H+=H2O+C
H++Cl-+HClO ,HCO3-+H+=H2O+C O2↑(或者Cl2+ HCO3-=CO2 ↑+Cl-+HClO );(5)无尾气处理装置;(6)35.8%(或35.75%)。
O2↑(或者Cl2+ HCO3-=CO2 ↑+Cl-+HClO );(5)无尾气处理装置;(6)35.8%(或35.75%)。
解析

 一本好题口算题卡系列答案
一本好题口算题卡系列答案