ΧβΡΩΡΎ»ί
AΘ°BΘ°CΘ°DΘ°EΈΣΚ§Ά§“Μ÷÷‘ΣΥΊΒΡ≥ΘΦϊΈο÷ ΓΘCΈο÷ ÷Μ”…“Μ÷÷‘ΣΥΊΉι≥…Θ§‘Ύ1ΗωCΖ÷Ή”÷––Έ≥…Ι≤ΦέΦϋΒΡΒγΉ” ΐ”κΖ÷Ή”÷–ΥυΚ§ΒγΉ” ΐ÷°±»ΈΣ3ΓΟ7ΓΘCΚΆEΨυΩ…”κ―θΤχ‘Ύ“ΜΕ®ΧθΦΰœ¬Ζ¥”Π…ζ≥…AΓΘ«κΜΊ¥π“‘œ¬Έ ΧβΘΚ?
Θ®1Θ©≥ΘΈ¬œ¬ΫΪΤχΧεBΆ®»κΥ°÷–ΖΔ…ζΖ¥”ΠΘ§…ζ≥…AΚΆDΘ§‘ρΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ ΓΘ
Θ®2Θ©–¥≥ωE”κ―θΤχΖ¥”Π…ζ≥…AΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®3Θ©DΚΆE…ζ≥…ΒΡΜ·ΚœΈο‘ΎΡ≥Έ¬Ε»œ¬Φ”»»Ζ÷ΫβΘ§Ά§ ±…ζ≥…ΝΫ÷÷―θΜ·ΈοΓΘ«“‘Ύ¥ΥΙΐ≥Χ÷–Θ§»τ”–0Θ°5 molΗΟΜ·ΚœΈοΆξ»ΪΖ¥”ΠΘ§ΉΣ“ΤΒγΉ” ΐΈΣ2 molΓΘ–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®4Θ©œ¬ΆΦΥυ Ψ «Ϋχ––ΡΨΧΩ”κD≈®»ή“ΚΖ¥”ΠΘ§≤ΔΦλ―ι…ζ≥…ΒΡΤχΧεΚΆΖ¥”ΠΒΡ»»–ß”ΠΒΡ Β―ιΉΑ÷ΟΘ§ΥϋΨΏ”–ΈόΈέ»ΨΘ°œ÷œσΟςœ‘Β»ΧΊΒψΓΘΨΏ÷ß ‘ΙήA÷–Υυ ΔΙΧΧεœ¬≤ψ «ΈόΥ°CaCl2Θ®Ής‘ΊΧε≤Μ≤ΈΦ”Ζ¥”ΠΘ©Θ§…œ≤ψ «Κλ»»ΒΡΡΨΧΩΓΘ Β―ι ±¬ΐ¬ΐΫΪD≈®»ή“ΚΒΈΒΫΡΨΧΩ…œΘ§Ζ¥”ΠΦ¥ΩΣ ΦΫχ––«“Ρή≥Λ ±ΦδΨγΝ“Ζ¥”ΠΓΘΔΌ–¥≥ωΡΨΧΩ”κD≈®»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ

ΔΎΗΟΖ¥”ΠΈΣ Θ®ΧνΓΑΈϋ»»Γ±ΜρΓΑΖ≈»»Γ±Θ©Ζ¥”ΠΓΘ
Δέ ‘ΙήBΡΎ≥ωœ÷ΒΡœ÷œσΈΣ ΓΘ
Δή‘Ύ Β―ιΝΌΫϋΫα χ ±Θ§ΖΔœ÷ΒΈΙή÷–ΒΡD≈®»ή“ΚΡ―“‘ΒΈœ¬ΓΘ»τœκ‘ΌΒΈ»κΦΗΒΈD≈®»ή“Κ ΙΡΨΧΩΖ¥”ΠΆξ»ΪΘ§ΉνΦρΒΞΒΡΑλΖ® « ΓΘ
Θ®1Θ©≥ΘΈ¬œ¬ΫΪΤχΧεBΆ®»κΥ°÷–ΖΔ…ζΖ¥”ΠΘ§…ζ≥…AΚΆDΘ§‘ρΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ ΓΘ
Θ®2Θ©–¥≥ωE”κ―θΤχΖ¥”Π…ζ≥…AΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®3Θ©DΚΆE…ζ≥…ΒΡΜ·ΚœΈο‘ΎΡ≥Έ¬Ε»œ¬Φ”»»Ζ÷ΫβΘ§Ά§ ±…ζ≥…ΝΫ÷÷―θΜ·ΈοΓΘ«“‘Ύ¥ΥΙΐ≥Χ÷–Θ§»τ”–0Θ°5 molΗΟΜ·ΚœΈοΆξ»ΪΖ¥”ΠΘ§ΉΣ“ΤΒγΉ” ΐΈΣ2 molΓΘ–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®4Θ©œ¬ΆΦΥυ Ψ «Ϋχ––ΡΨΧΩ”κD≈®»ή“ΚΖ¥”ΠΘ§≤ΔΦλ―ι…ζ≥…ΒΡΤχΧεΚΆΖ¥”ΠΒΡ»»–ß”ΠΒΡ Β―ιΉΑ÷ΟΘ§ΥϋΨΏ”–ΈόΈέ»ΨΘ°œ÷œσΟςœ‘Β»ΧΊΒψΓΘΨΏ÷ß ‘ΙήA÷–Υυ ΔΙΧΧεœ¬≤ψ «ΈόΥ°CaCl2Θ®Ής‘ΊΧε≤Μ≤ΈΦ”Ζ¥”ΠΘ©Θ§…œ≤ψ «Κλ»»ΒΡΡΨΧΩΓΘ Β―ι ±¬ΐ¬ΐΫΪD≈®»ή“ΚΒΈΒΫΡΨΧΩ…œΘ§Ζ¥”ΠΦ¥ΩΣ ΦΫχ––«“Ρή≥Λ ±ΦδΨγΝ“Ζ¥”ΠΓΘΔΌ–¥≥ωΡΨΧΩ”κD≈®»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ

ΔΎΗΟΖ¥”ΠΈΣ Θ®ΧνΓΑΈϋ»»Γ±ΜρΓΑΖ≈»»Γ±Θ©Ζ¥”ΠΓΘ
Δέ ‘ΙήBΡΎ≥ωœ÷ΒΡœ÷œσΈΣ ΓΘ
Δή‘Ύ Β―ιΝΌΫϋΫα χ ±Θ§ΖΔœ÷ΒΈΙή÷–ΒΡD≈®»ή“ΚΡ―“‘ΒΈœ¬ΓΘ»τœκ‘ΌΒΈ»κΦΗΒΈD≈®»ή“Κ ΙΡΨΧΩΖ¥”ΠΆξ»ΪΘ§ΉνΦρΒΞΒΡΑλΖ® « ΓΘ
Θ®1Θ©3NO2+H2O=2H++2NO-3+NO Θ®2Θ©4NH3+5O2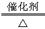 4NO+6H2O
4NO+6H2O
Θ®3Θ©NH4NO3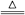 2H2O+N2OΓϋ?
2H2O+N2OΓϋ?
Θ®4Θ©ΔΌC+4HNO3Θ®≈®Θ©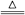 CO2Γϋ+4NO2Γϋ+2H2O
CO2Γϋ+4NO2Γϋ+2H2O
ΔΎΖ≈»» Δέ ‘ΙήΡΎ”–ΚλΉΊ…ΪΤχΧε…ζ≥…Θ§ ·Μ“Υ°±δΜκΉ«
Δή“ΓΕ· ‘ΙήBΘ§ Ι…œ≤ΩΒΡΤχΧε»ή”Ύ ·Μ“Υ°
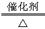 4NO+6H2O
4NO+6H2OΘ®3Θ©NH4NO3
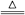 2H2O+N2OΓϋ?
2H2O+N2OΓϋ?Θ®4Θ©ΔΌC+4HNO3Θ®≈®Θ©
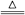 CO2Γϋ+4NO2Γϋ+2H2O
CO2Γϋ+4NO2Γϋ+2H2OΔΎΖ≈»» Δέ ‘ΙήΡΎ”–ΚλΉΊ…ΪΤχΧε…ζ≥…Θ§ ·Μ“Υ°±δΜκΉ«
Δή“ΓΕ· ‘ΙήBΘ§ Ι…œ≤ΩΒΡΤχΧε»ή”Ύ ·Μ“Υ°
¬‘

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ
ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ ≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗ
≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
 ΧεXΘ§ΫΪXΉω»γœ¬ΆΦΥυ ΨΒΡ Β―ιΘΚ
ΧεXΘ§ΫΪXΉω»γœ¬ΆΦΥυ ΨΒΡ Β―ιΘΚ
 ≥ωAΚΆBΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
≥ωAΚΆBΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ ΓΘ
ΓΘ