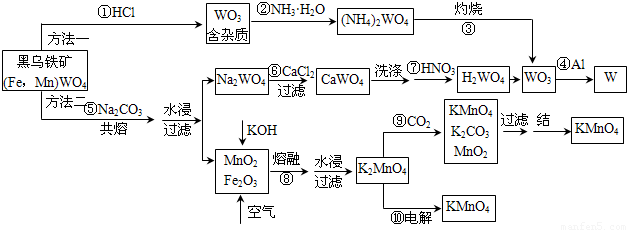
��Ŀ����
(4��)��ҵ����MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷�Ϊ�������У���һ����MnO2��KOH�����飬���ȣ��ڿ����м������ۻ������������裬��ȡK2MnO4���ڶ�����K2MnO4��Ũ��Һ�ö��Ե缫���е�⣬�������ϵõ�KMnO4���������ϵõ�KOH�����K2MnO4��Ũ��Һʱ�����������ĵ缫��Ӧʽ��
������ �������� ��
�����ܷ�Ӧ����ʽ�� ��

�����������������ϵõ�KMnO4���������ϵõ�KOH��������ˮ�ĵ���ƽ�ⱻ�ƻ���������2H2O ��2e�D=H2��2OH�D ;������MnO42�D�De�D=MnO42�D
�ܷ�Ӧ��2K2MnO4��2H2O=2KMnO4��2KOH��H2��

����ѧѡ��2����ѧ�뼼����(15��)
��ҵ��Ϊ��ʹԭ�Ϻ������õ���ֵ����ã���������ѭ��������
I�������Ȼ�ѧѭ�������ܻ�����Դì�ܡ�����о����֣�����������������(MnFe2O4)�������Ȼ�ѧѭ���ֽ�ˮ���⡣MnFe2O4���Ʊ���
��֪Fe3+��Mn2+������pH���ұ���ʾ��
|
| ��ʼ���� | ��ȫ���� |
| Fe3+ | 2.7 | 4.2 |
| Mn2+ | 8.3 | 10.4 |
(1)�˹�����������Ͷ��ԭ��Fe(NO3)3��Mn(NO3)2�����ʵ���֮��ӦΪ ��
(2)����pH�IJ�����m��ֵΪ ��
II����MnFe2O4�Ȼ�ѧѭ����ȡ������
(3)���Ͽ�֪��H2ȼ�յ��Ȼ�ѧ����ʽ�� ��
(4)���Ȼ�ѧѭ����ȡ�������ŵ��� (����ĸ���)��
A�����̼�����Ⱦ B�����Ͽ�ѭ��ʹ��
C�������������ڲ�ͬ�������ɣ���ȫ������
III����ҵ�Ͽ���H2��HClͨ����ͼ��ѭ��������ȡ̫���ܲ��ϸߴ��衣
��Ӧ��
��Ӧ�ڣ�
��5����ͼ�У�������ÿһ�ִε�Ͷ�������У���Ԫ��û����ʧ����Ӧ����HCl�������ʺͷ�Ӧ����H2�������ʾ�Ϊ75����������һ�ִε������У��貹��Ͷ��HCl��H2��������� ��
����ѧѡ��2����ѧ�뼼����(15��)
��ҵ��Ϊ��ʹԭ�Ϻ������õ���ֵ����ã���������ѭ��������
I�������Ȼ�ѧѭ�������ܻ�����Դì�ܡ�����о����֣�����������������(MnFe2O4)�������Ȼ�ѧѭ���ֽ�ˮ���⡣MnFe2O4���Ʊ���
��֪Fe3+��Mn2+������pH���ұ���ʾ��
| | ��ʼ���� | ��ȫ���� |
| Fe3+ | 2.7 | 4.2 |
| Mn2+ | 8.3 | 10.4 |
(2)����pH�IJ�����m��ֵΪ ��
II����MnFe2O4�Ȼ�ѧѭ����ȡ������
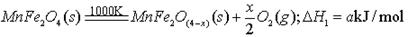
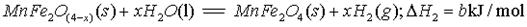
(3)���Ͽ�֪��H2ȼ�յ��Ȼ�ѧ����ʽ�� ��
(4)���Ȼ�ѧѭ����ȡ�������ŵ���
 (����ĸ���)��
(����ĸ���)��A�����̼�����Ⱦ
 B�����Ͽ�ѭ��ʹ��
B�����Ͽ�ѭ��ʹ��C�������������ڲ�ͬ�������ɣ���ȫ������
III����ҵ�Ͽ���H2��HClͨ����ͼ��ѭ��������ȡ̫���ܲ��ϸߴ��衣
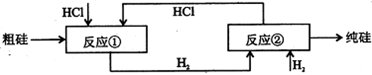
��Ӧ��
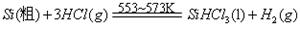
��Ӧ�ڣ�
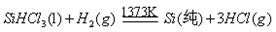
��5����ͼ�У�������ÿһ�ִε�Ͷ�������У���Ԫ��û����ʧ����Ӧ����HCl�������ʺͷ�Ӧ����H2�������ʾ�Ϊ75����������һ�ִε������У��貹��Ͷ��HCl��H2��������� ��
����ѧѡ��2����ѧ�뼼����(15��)
��ҵ��Ϊ��ʹԭ�Ϻ������õ���ֵ����ã���������ѭ��������
I�������Ȼ�ѧѭ�������ܻ�����Դì�ܡ�����о����֣�����������������(MnFe2O4)�������Ȼ�ѧѭ���ֽ�ˮ���⡣MnFe2O4���Ʊ���

��֪Fe3+��Mn2+������pH���ұ���ʾ��
|
|
��ʼ���� |
��ȫ���� |
|
Fe3+ |
2.7 |
4.2 |
|
Mn2+ |
8.3 |
10.4 |
(1)�˹�����������Ͷ��ԭ��Fe(NO3)3��Mn(NO3)2�����ʵ���֮��ӦΪ ��
(2)����pH�IJ�����m��ֵΪ ��
II����MnFe2O4�Ȼ�ѧѭ����ȡ������


(3)���Ͽ�֪��H2ȼ�յ��Ȼ�ѧ����ʽ�� ��
(4)���Ȼ�ѧѭ����ȡ�������ŵ��� (����ĸ���)��
A�����̼�����Ⱦ B�����Ͽ�ѭ��ʹ��
C�������������ڲ�ͬ�������ɣ���ȫ������
III����ҵ�Ͽ���H2��HClͨ����ͼ��ѭ��������ȡ̫���ܲ��ϸߴ��衣

��Ӧ��
��Ӧ�ڣ�
��5����ͼ�У�������ÿһ�ִε�Ͷ�������У���Ԫ��û����ʧ����Ӧ����HCl�������ʺͷ�Ӧ����H2�������ʾ�Ϊ75����������һ�ִε������У��貹��Ͷ��HCl��H2��������� ��