��Ŀ����
��1��0.02 mol��L-1��HCN��Һ��0.02 mol��L-1NaCN��Һ�������ϣ���֪�û����Һ��c(Na+)> c(CN-)���á�>��<��=���������
����Һ��c(OH -) c(H +) �� c(HCN) c(CN-)
��2�������£���������Һ�е���Ba(OH)2��Һ��SO42- �պ���ȫ����ʱ����Һ
pH 7�����á�>��<��=��������գ���Ӧ�����ӷ���ʽΪ__________________________________��
��3��BaCO3�ֱ���룺�� 30mLˮ ��10mL0.2mol/LNa2CO3��Һ ��50mL 0.01mol/L
�Ȼ�����Һ ��100mL 0.01mol/L�������ܽ�����Һ���͡���ȷ������Һ��
Ba2+��Ũ���ɴ�С��˳��Ϊ��___________________________________________��
��1���� > ��2�֣��� �� > ��2�֣���
��2��> ��2�֣��� Al3++2SO42-+2Ba2++4OH-= 2BaSO4��+ AlO2- ��2�֣���
(3) �ۢܢ٢� ��2�֣�
����:��1�����ݵ���غ��֪c(Na��)��c(H��)��c(OH��)��c(CN��)�����Ե�c (Na+)> c(CN-)ʱ��c(OH -)��c(H+)����˵����Һ�Լ��ԣ�����HCN�ĵ���̶�С��NaCN��ˮ��̶ȣ����c(HCN) ��c(CN-)��
��2��������KAl(SO4)2����SO42- �պ���ȫ����ʱ����Ҫ2molBa(OH)2��Һ�����ʱ������ǡ������KAlO2��KAlO2ˮ���Լ��ԣ���ӦʽΪAl3++2SO42-+2Ba2++4OH-= 2BaSO4��+ AlO2-��
��3��BaCO3(s) Ba2��(aq)��CO32��(aq)̼�ᱵ������Һ�д����ܽ�ƽ�⣬�����ܶȻ�������֪CO32��Ũ��Խ������Ũ�Ⱦ�ԽС��ƽ�����ң������������ӵ�Ũ�ȣ�������ȷ��˳���Ǣۢܢ٢�

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д��ڴ��������£������ͨ��������Ӧ�����飬��Ӧԭ�����£�
�� CH3COOH��2H2��CH3CH2OH��H2O �� CH3CH2OH��H2��CH3CH3��H2O
�� CH3COOH��3H2��CH3CH3��2H2O �ڷ�Ӧ������������Ҵ�����������������Ϊ�о��ֱ��˼�Mo(��)�Ͳ���Mo������Ni(��)������Ч�ܣ�ij�����ص��������£�
| ����ת���� | δ��Ӧ�ĺ��������ﺬ�� | |||||
| ���� | �Ҵ� | |||||
| �¶� / �� | Mo16Ni6 | Ni6 | Mo16Ni6 | Ni6 | Mo16Ni6 | Ni6 |
| 240 | 87.2 | 21.8 | 0.53 | 3.24 | 1.03 | 0.14 |
| 260 | 89.1 | 27.2 | 0.45 | 3.02 | 0.99 | 0.19 |
������������ȷ����
A�������٢ڢ�����Ӧ�����ڼӳɷ�Ӧ B�������٢ڢ�����Ӧ�����ڻ�ԭ��Ӧ
C���������������Ӧ260���240����и����� D��Mo16Ni6�����ܱ�Ni6��
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5��![]() ����1��
����1��![]() ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS>CO2
C����ͬѹǿ�·е㣺C2H5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4��![]() -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� ��
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
����������ȩ��״����Ϸ�Ӧʵ���д���������������Ϊ ��
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |
�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��
�ڴ��������������ͨ��������Ӧ�����飬��Ӧԭ�����£�
��CH3COOH+2H2��CH3CH2OH+H2O
��CH3CH2OH+H2��CH3CH3+H2O
��CH3COOH+3H2��CH3CH3+2H2O
�ڷ�Ӧ������������Ҵ�����������������Ϊ�о��ֱ��˼�Mo���⣩�Ͳ���Mo������Ni������������Ч�ܣ�ij�����ص��������£�
|
|
����ת���� |
δ��Ӧ�ĺ��������ﺬ�� |
||||
|
���� |
�Ҵ� |
|||||
|
�¶�/�� |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
|
240 |
87.2 |
21.8 |
0.53 |
3.24 |
1.03 |
0.14 |
|
260 |
89.1 |
27.2 |
0.45 |
3.02 |
0.99 |
0.19 |
����˵������ȷ���� �� ��
A�������٢ڢ�����Ӧ�����ڼӳɷ�Ӧ
B�������٢ڢ�����Ӧ�����ڻ�ԭ��Ӧ
C���������������Ӧ260���240���½��и�����
D��Mo16Ni6�����ܱ�Ni6��
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5�� ����1��
����1��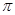 ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS>CO2
C����ͬѹǿ�·е㣺C2H5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4��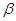 -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� ��
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
|
��������/mol |
0.01 |
0.02 |
0.03 |
0.05 |
0.1 |
0.15 |
0.2 |
0.6 |
|
����% |
87.3 |
88.2 |
90.3 |
94.2 |
92.9 |
93.1 |
91.8 |
92.3 |
|
ת����% |
89.7 |
92.1 |
93.9 |
98.9[��Դ:Zxxk.Com] |
94.9 |
95.7 |
93.9 |
94.3 |
����������ȩ��״����Ϸ�Ӧʵ���д���������������Ϊ ��
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
|
��� |
ȩ |
�� |
ת����% |
����% |
|
1 |
���ǻ�����ȩ |
�״� |
94.3 |
89.6 |
|
2 |
���ǻ�����ȩ |
�״� |
93.6 |
88.7 |
|
3 |
���ȱ���ȩ |
�״� |
93.1 |
87.3 |
|
4 |
����������ȩ |
�״� |
54.2 |
34.1 |
|
5 |
����������ȩ |
�״� |
89.9 |
79.5 |
|
6 |
����������ȩ |
�״� |
65.7 |
41.9 |

�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��