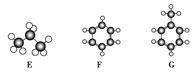
��Ŀ����
��1�����и����е������л����������ͬ�����ʡ�ͬϵ���ͬ���칹��ȣ����ж�����֮��Ĺ�ϵ
��2��������Ͷ��� ��
��1����ϩ�ͻ����� ��
��2��֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽ ��
��3��д����ȩ��Һ��������������Һ���ȵĻ�ѧ����ʽ�� ��
��4��д��1,3-����ϩ���嵥�ʷ���1,4-�ӳɵķ�Ӧ����ʽ
��5��д�����Ҵ�һ����������Ļ�ѧ����ʽ
��10�֣�
��1����ͬϵ�1�֣� ��ͬ���칹�壨1�֣�
��2��3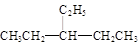 ��2�֣�
��2�֣�
��3��CH3CHO��2[Ag(NH3)2]����2OH�� CH3COO����NH
CH3COO����NH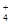 ��2Ag����3NH3��H2O��2�֣�
��2Ag����3NH3��H2O��2�֣�
��4��CH2=CH��CH=CH2��Br2��CH2BrCH=CHCH2Br��2�֣�
��5��CH3CH2OH+HBr  CH3CH2Br+H2O��2�֣�
CH3CH2Br+H2O��2�֣�
�����������������2�����������ʽ��C5H12���������ʽ��C4H10������Ϊͬϵ�
��1����ϩ����ʽ��C6H12�����������ʽ��C6H12����Ϊͬ���칹�塣
��2��֧��ֻ��һ���һ����������һ�����̼ԭ�ӱ����С��3(���κ�һ������)����ʽ����С�������ǣ�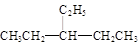 ��
��
��3����ȩ��Һ��������������Һ���ȵĻ�ѧ����ʽ��CH3CHO��2[Ag(NH3)2]����2OH�� CH3COO����NH
CH3COO����NH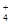 ��2Ag����3NH3��H2O��
��2Ag����3NH3��H2O��
��4��1,3-����ϩ���嵥�ʷ���1,4-�ӳɵķ�Ӧ����ʽCH2=CH��CH=CH2��Br2��CH2BrCH=CHCH2Br
��5�����Ҵ�һ����������Ļ�ѧ����ʽCH2=CH��CH=CH2��Br2��CH2BrCH=CHCH2Br��
���㣺ȷ�ı����л���Ļ�ѧ���

ij��ϩ���������ļӳɲ���Ϊ��(CH3 )2CHCH2CH3 ,�������˵����ȷ����
| A�����������1��1���������� |
| B��ԭ��ϩ��ֻ������3�ֲ�ͬ�ṹ |
| C��1mol�ӳɲ���ȼ������6.5mol���� |
| D��ԭϩ�������ʽ��C3H6����һ����Ϊͬϵ�� |
ij���Ľṹ��ʽΪ �������ܾ��е������� ( )
�������ܾ��е������� ( )
| A������ʹ��ˮ��ɫ��������ʹ���Ը��������Һ��ɫ |
| B��������ʹ��ˮ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ |
| C��������ˮ��Ҳ�������л��ܼ� |
| D���ܷ����ӳɷ�Ӧ��һ���������������������ʵ����������ӳ� |


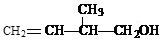
 Fe2++SO42-
Fe2++SO42-