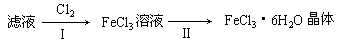��Ŀ����
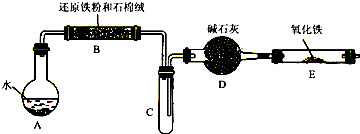
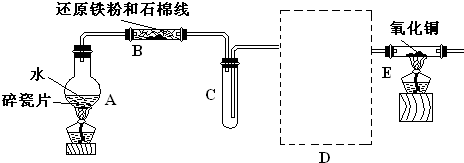
��12�֣�ѧ��������ͼ����װ�ý��С�����ˮ������Ӧ����ʵ�飬�����ò����һ����ȡFeCl3��6H2O���塣��ͼ�мгּ�β������װ�þ�����ȥ��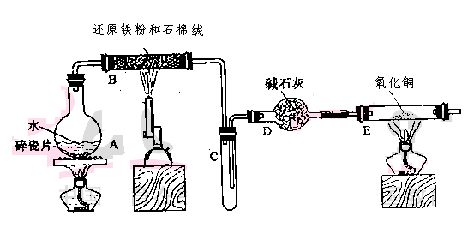
��1���ش��������⣺�����������������ʣ�����ȥ���������л��е����ۿ���ѡ�õ��Լ�Ϊ
__ _ ������ţ���
| A��ϡ���� | B������������Һ | C��Ũ���� | D��FeCl3��Һ |
��2����Ӧ������װ��B�з�����Ӧ�Ļ�ѧ����ʽ��_______________ ____��
Dװ�õ����ã� ��
��3����С��ѧ����B�з�Ӧ��IJ���������������ᣬ���ˣ���������Һ��ȡFeCl3��6H2O���壬����������£�
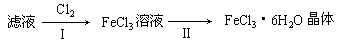
�������ӷ���ʽ��ʾ����I��ͨ��Cl2������ ��
��Ϊ�˼���ijδ֪��Һ�Ƿ���FeCl2��Һ��ͬѧ�����������ʵ�鷽������֤����
��һ֧װ�и�δ֪��Һ���Թ�����ͨ���������ٵμ�KSCN��Һ����Һ���ֺ�ɫ��֤����δ֪��Һ��FeCl2��Һ������Ϊ�˷����Ƿ���� (���������������)��
(ÿ��2��) (1) B 2Al+2OH-+2H2O=2AlO2-+3H2��
(2) 3Fe + 4H2O Fe3O4 + 4H2 . ��ȥH2�е�ˮ����
Fe3O4 + 4H2 . ��ȥH2�е�ˮ����
��3����Cl2 + 2Fe2+ =2Fe3+ +2 Cl- �ڡ�������
����

��ϰ��ϵ�д�
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�
�����Ŀ