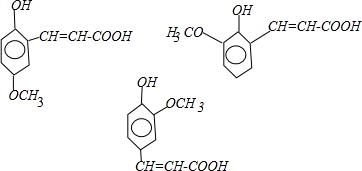
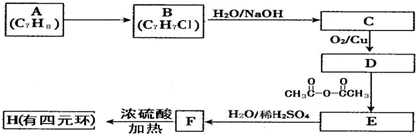
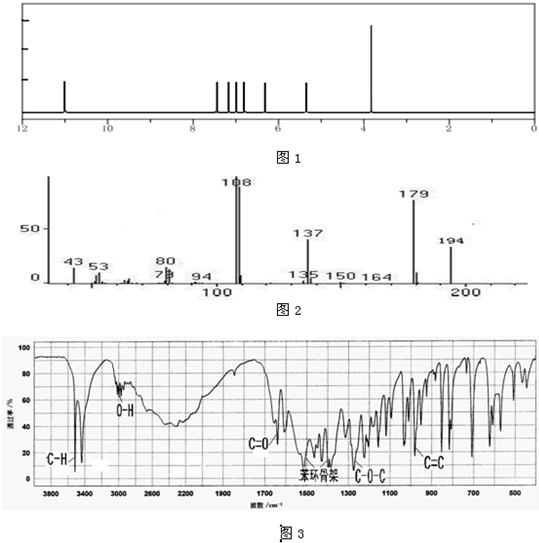
��Ŀ����
Ϊ̽���������ȡ����Ӧ��������ͼװ�â��������ʵ�飺

��һ�����ı����������ƿ�У�ͬʱ����������м��3��5 min����װ��AgNO3��Һ����ƿ����dz��ɫ�ij������ɣ���֤�������巢����ȡ����Ӧ��
��1��װ��I�ТٵĻ�ѧ����ʽΪ
_______________________________________________________��
_______________________________________________________��
��2�����г����ܵ�������________________________________________________��
��3����ƿ�����ɵĺ��ɫ��״Һ�εijɷ���__________________________��Ҫ��õ������IJ������____________�Լ�ϴ�ӡ�ϴ�Ӻ�����ƷӦʹ�õ�������_______________��
��4������ʵ��ʱ���ҹ۲쵽��ƿ��Һ����ڲ��к���ɫ����ӵ������ݳ�����������ȳ�ȥ����ɫ���壬������֤��ƿ�еIJ��ԭ����___________________________________��
��5����ͬѧ�����ͼ��ʾװ�â�������ijЩ�Լ���ɸ�ʵ�顣��ѡ�õ��Լ��ǣ�����Һ�壻Ũ�������������Һ����������Һ�����Ȼ�̼��
a��������___________________________________
b�е��Լ���_________________________________
�Ƚ�����װ�ã�װ�â����Ҫ�ŵ��ǣ���������㼴�ɣ�
��____________________________________________
��____________________________________________
��11�֣�
��1��2Fe��3Br2===2FeBr3
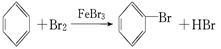
��2����������������
��3��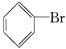 ��Br2 NaOH��Һ ��Һ©��
��Br2 NaOH��Һ ��Һ©��
��4���ӷ�����������Ҳ������������Һ��Ӧ����AgBrdz��ɫ����
��5����ֹ������ȫװ�� �������Ȼ�̼��
��Ҫ�ŵ��ǣ�
�ٷ�ֹ������ �ڿ��Կ��Ʒ�Ӧ���г̶ȡ� �۱������ʸ��š� �ܷ�ֹ��Ⱦ����
��������
�����������1������������Ӧ�����廯����2Fe+3Br2 �T2FeBr3������Һ�����廯���Ĵ������������屽���廯�⣺C6H6+Br2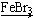 C6H5Br+HBr���ʴ�Ϊ��2Fe+3Br2�T2FeBr3��C6H6+Br2
C6H5Br+HBr���ʴ�Ϊ��2Fe+3Br2�T2FeBr3��C6H6+Br2 C6H5Br+HBr��
C6H5Br+HBr��
��2����ӦΪ���ȷ�Ӧ���������ûӷ��������嵥�����裬��ֹ�Բ���ĸ��ţ��������ܵ�����Ϊ����������������
��3����Ӧ���ɵ����������廥�ܳʺ��ɫ��״Һ�Ρ�����NaOH��Һ�Լ�ϴ�ӣ��÷�Ӧ��������ˮ�����ʣ��ٽ��з�Һ���ʴ�Ϊ ��Br2 NaOH��Һ ��Һ©����
��Br2 NaOH��Һ ��Һ©����
��4����֤������Ҫ���ӣ�����ӷ�����������Ҳ������������Һ��Ӧ����AgBrdz��ɫ������
��5����ͼ����Ϣ��֪��֧���Ƕ̽��̳�a�ĵ������Ƿ�ֹ������ȫװ�á�B��Ҫ��ȥBr2���Լ�Ϊ�������Ȼ�̼���������Ϸ�����װ�â����Ҫ�ŵ��Ǣٷ�ֹ�������ڿ��Կ��Ʒ�Ӧ���г̶ȣ��۱������ʸ��ţ��ܷ�ֹ��Ⱦ������
���㣺�������� ʵ����Ƽ�̽��
������������Ҫ�����˱�������ʵ�飬���շ�Ӧ��ԭ���������ʵ������Լ������ķ����ǽ���Ĺؼ���

 ������״Ԫ���Ծ�ϵ�д�
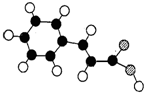
������״Ԫ���Ծ�ϵ�д�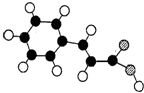 ��1������ᣨE���ķ��ӽṹģ������ͼ��ʾ��ͼ��������֮�����߱�ʾ������˫������
��1������ᣨE���ķ��ӽṹģ������ͼ��ʾ��ͼ��������֮�����߱�ʾ������˫������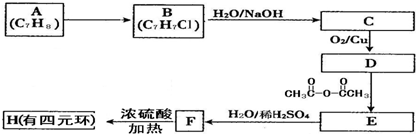
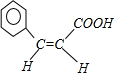
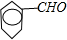
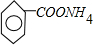 +2Ag��+3NH3+H2O
+2Ag��+3NH3+H2O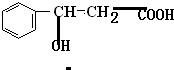
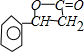 +H2O
+H2O