��Ŀ����
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
(1)ʵ���ã�1 g�״��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ__________________________________
(2)��֪��ӦCH3��CH3(g)�D��CH2=CH2(g)��H2(g)���йػ�ѧ���ļ������¡�
�Լ���÷�Ӧ�ķ�Ӧ��___________________________
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)=CH3COOH(l)���ʱ䦤H��________��
��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��1/2O2(g)=H2O(l)
��H3����285.8 kJ��mol��1
(1)ʵ���ã�1 g�״��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ__________________________________
(2)��֪��ӦCH3��CH3(g)�D��CH2=CH2(g)��H2(g)���йػ�ѧ���ļ������¡�
| ��ѧ�� | C��H | C=C | C��C | H��H |
| ����/kJ��mol��1 | 414.4 | 615.3 | 347.4 | 435.3 |
�Լ���÷�Ӧ�ķ�Ӧ��___________________________
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)=CH3COOH(l)���ʱ䦤H��________��
��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��1/2O2(g)=H2O(l)
��H3����285.8 kJ��mol��1
(1)2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l)
����H����1 452.8 kJ��mol��1
(2)��125.6 kJ��mol��1
(3)��488.3 kJ��mol��1
����H����1 452.8 kJ��mol��1
(2)��125.6 kJ��mol��1
(3)��488.3 kJ��mol��1
(2)��H��6E(C��H)��E(C��C)��[E(C=C)��4E(C��H)��E(H��H)]��6��414.4��347.4��(615.3��4��414.4��435.3)����125.6(kJ��mol��1)��
(3)��H��2����H2��2����H3����H1��2��(��393.5)��2��(��285.5)��(��870.3)����488.3(kJ��mol��1)��
(3)��H��2����H2��2����H3����H1��2��(��393.5)��2��(��285.5)��(��870.3)����488.3(kJ��mol��1)��

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
�����Ŀ
 HCOONa+H2O�����й�˵����ȷ����
HCOONa+H2O�����й�˵����ȷ���� 
 NaClO+NaCl+H2O
NaClO+NaCl+H2O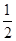 O2(g)=CO(g)����H����Q1kJ��mol��1��C(s)��O2(g)=CO2(g)����H����Q2 kJ��mol��1���й�������Ӧ������������ǣ� ��
O2(g)=CO(g)����H����Q1kJ��mol��1��C(s)��O2(g)=CO2(g)����H����Q2 kJ��mol��1���й�������Ӧ������������ǣ� ��