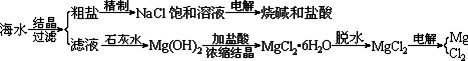��Ŀ����
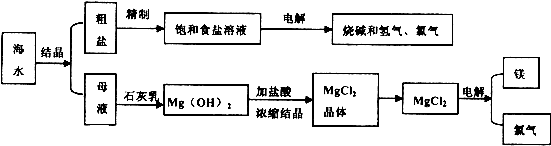
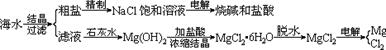
��ˮ��ȡ֮�����Ļ���ԭ����Դ�⣬�Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϡ������ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ��
ͼ2-10
�Իش��������⣺
(1)�����к���Ca2+��Mg2+��![]() �����ʣ�����ʱ�����Լ�Ϊ��A.���B.BaCl2��Һ��C.NaOH��Һ��D.Na2CO3��Һ�������Լ���˳���ǣ�_______________________________________��
�����ʣ�����ʱ�����Լ�Ϊ��A.���B.BaCl2��Һ��C.NaOH��Һ��D.Na2CO3��Һ�������Լ���˳���ǣ�_______________________________________��
(2)��ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ����ķ�ӦΪ_____________________�����Դ���������ĵ缫������ҺpH__________�������䡱��С��������1 mol���ӵĵ���Ϊ96
(3)��MgCl2��6H2O������ˮ����ˮMgCl2ʱ��MgCl2��6H2O������_________�����м�����ˮ���������������_____________________________��
(4)�����ˮMgCl2���õ�þ��������������____________��������ȴ��
A.H2 B.N
˼·������(1)��С�������ӳ����⡣����ԭ�����ڳ�ȥCa2+��Mg2+��![]() ʱ�����ܴ����������ӡ����ԣ������Ĺؼ��ǰ��պü������ӵ�˳��Ba2+������
ʱ�����ܴ����������ӡ����ԣ������Ĺؼ��ǰ��պü������ӵ�˳��Ba2+������![]() ֮ǰ���룻 ��
֮ǰ���룻 ��![]() ��OH��������H+֮ǰ���룬����B��C�����Ⱥ�D��A�����ȷ�ǰ���ֱ������B��C֮���������������
��OH��������H+֮ǰ���룬����B��C�����Ⱥ�D��A�����ȷ�ǰ���ֱ������B��C֮���������������
(2)��ⱥ��ʳ��ˮʱ�����������������������ķ�Ӧ�ǣ�2Cl-��2e-![]() Cl2���븺�����������������ķ�Ӧ�ǣ�2H+��2e-
Cl2���븺�����������������ķ�Ӧ�ǣ�2H+��2e-![]() H2��H+�������ģ�ʹ����Һ��c��OH-������pH�������������Cl2��H2������Ҫȷ��ԭ��ҺŨ�Ȳ��䣬ֻ������ϵ��ͨ��һ������HCl�����Բ�����ʧ��H��Clԭ�ӡ��״����Ǽ������ᣬʹ��ҺŨ�ȱ�С��
H2��H+�������ģ�ʹ����Һ��c��OH-������pH�������������Cl2��H2������Ҫȷ��ԭ��ҺŨ�Ȳ��䣬ֻ������ϵ��ͨ��һ������HCl�����Բ�����ʧ��H��Clԭ�ӡ��״����Ǽ������ᣬʹ��ҺŨ�ȱ�С��
(3)����ˮ��ƽ��MgCl2��H2O![]() Mg��OH��Cl��HCl������С�
Mg��OH��Cl��HCl�������
(4)þ���������뵪���������Ͷ�����̼��Ӧ������ֻ������������ȴ��
�𰸣�(1)BCDA��CBDA (2)2Cl-��2e-![]() Cl2 ��� 4 480 ����Һ��ͨ��һ������HCl���� (3)HCl ��ֹMg2+ˮ�� (4)A
Cl2 ��� 4 480 ����Һ��ͨ��һ������HCl���� (3)HCl ��ֹMg2+ˮ�� (4)A

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�