جâؤ؟ؤعبف
بًت؟»¯ر§¼زأ×ہصزٍ؛د³ةDDTسع1948ؤê»ٌµأإµ±´¶û½±،£شعµع¶´ختہ½ç´َص½ضذµؤ1944ؤ꣬أہ¹ْشعزâ´َہûµؤذي¶àت؟±ّزٍت¹سأDDTہ´ئثأً´«ب¾°كصîةث؛®ء÷ذذ²،بثةيةدµؤت×س¶ّ±ـأâة¥ةْ،£ءھ؛د¹ْتہ½çخہةْ×éض¯شّئہ¼غثµ£؛،°µ¥¶ہ´سإ±¼²²،صك£¬DDT؟ةؤـصü¾بءث5 000حٍةْأü،£،±µ«½ّز»²½µؤ¹غ²ى؛حرذ¾؟±يأ÷£¬DDTتاز»ضضؤر½µ½âµؤسذ¶¾»¯؛دخ½ّبëبثجهؤع؟ةزئًآذشضذ¶¾،£خز¹ْزرسع1983ؤêح£ض¹ةْ²ْ؛حت¹سأ،£
(1)ة±³و¼ء،°1605،±±¾ةي¶شبثذَ¶¾ذشش¶ا؟سعDDT£¬µ«DDTزر±»½ûسأ£¬¶ّ،°1605،±ةذخ´½ûسأ£¬صâتاخھت²أ´£؟________________________________________________________________،£

سة½ل¹¹·ضخِضھ£؛،°1605،±شعت¹سأ¹³جضذ£¬²»ؤـسë________خïضت»ى؛دت¹سأ£¬·ٌشٍ»لزٍ________،£
(2)DDT؟ةزشسأµçت¯خھشءد¾زشدآح¾¾¶¶ّضئµأ£؛

¢ظذ´³ِ¢ـ¢ف¢ق²½·´س¦µؤ»¯ر§·½³جت½،£
¢عDDTسذ¶¾ذش£¬¹ْ¼تةدزر½ûض¹ت¹سأ£¬زٍخھثüشع¶¯خïجهؤع×ھ»¯خھز»ضضت¹¶¯خïةْ³¤ت§µ÷µؤخïضت(½ل¹¹¼ٍت½بçدآ)،£شعتµرéتزز²؟ةتµدضصâضض×ھ»¯£¬±يت¾صâضض×ھ»¯µؤ»¯ر§·½³جت½خھ____________________________________________________________،£
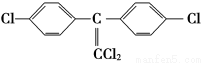
(1)،°1605،±شع»·¾³ضذ½µ½âأ¸×÷سأدآز×´سP،ھO´¦½µ½âخھ¶¾ذشذ،،¢²»²ذ´وµؤخïضت(20جى؟ةدûت§ز»°ë)£¬¶ّDDT²»ز×½µ½â£¬ثü³¤ئعضحءôسع¶¯خïجه»ٍت³خïء´ؤع(دàثئدàبـ)£¬خ£؛¦ذش´َ،،¼îذش،،ث®½â¶ّت§ذ§
(2)¢ظCHCH£«H2O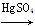 CH3CHO£»
CH3CHO£»
CH3CHO£«3Cl2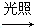 CCl3CHO£«3HCl£»
CCl3CHO£«3HCl£»
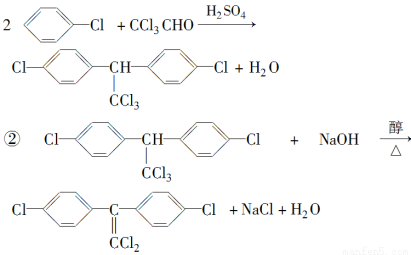
،¾½âخِ،؟(1)،°1605،±·ض×سضذ´وشعسëُ¥»ùہàثئµؤ¹ظؤـحإ£¬ئنشع¼îذشجُ¼دآبفز×ث®½â¶ّت§ذ§،£(2)·ضخِ؛د³ةDDTµؤح¾¾¶½ل؛دسذ»ْضھت¶£¬؛ـبفز×حئ³ِ´ً°¸،£

 ³ُضذر§زµ؟¼تشµ¼سëء·دµءذ´ً°¸
³ُضذر§زµ؟¼تشµ¼سëء·دµءذ´ً°¸¶¼×أرتاز»ضضضطزھµؤاه½àب¼ءد£¬ز²؟ةجو´ْ·ْہû°؛×÷ضئہن¼ءµب£¬¶ش³ôرُ²مخقئئ»µ×÷سأ،£¹¤زµةد؟ةہûسأأ؛µؤئّ»¯²ْخï(ث®أ؛ئّ)؛د³ة¶¼×أر،£
اë»ط´ًدآءذختجâ£؛
(1)أ؛µؤئّ»¯µؤض÷زھ»¯ر§·´س¦·½³جت½خھ£؛______________________________________،£
(2)أ؛µؤئّ»¯¹³جضذ²ْةْµؤسذ؛¦ئّجهH2SسأNa2CO3بـز؛خüتص£¬ةْ³ةء½ضضثلت½رخ£¬¸أ·´س¦µؤ»¯ر§·½³جت½خھ£؛____________________________________________________________،£
(3)ہûسأث®أ؛ئّ؛د³ة¶¼×أرµؤب²½·´س¦بçدآ£؛
¢ظ2H2(g)£«CO(g)  CH3OH(g)¦¤H£½£90.8 kJ،¤mol£1
CH3OH(g)¦¤H£½£90.8 kJ،¤mol£1
¢ع2CH3OH(g)  CH3OCH3(g)£«H2O(g)¦¤H£½£23.5 kJ،¤mol£1
CH3OCH3(g)£«H2O(g)¦¤H£½£23.5 kJ،¤mol£1
¢غCO(g)£«H2O(g)  CO2(g)£«H2(g)¦¤H£½£41.3 kJ،¤mol£1
CO2(g)£«H2(g)¦¤H£½£41.3 kJ،¤mol£1
×ـ·´س¦3H2(g)£«3CO(g)  CH3OCH3(g)£«CO2(g)µؤ¦¤H£½________£»
CH3OCH3(g)£«CO2(g)µؤ¦¤H£½________£»
ز»¶¨جُ¼دآµؤأـ±صبفئ÷ضذ£¬¸أ×ـ·´س¦´ïµ½ئ½؛⣬زھجل¸كCOµؤ×ھ»¯آت£¬؟ةزش²ةب،µؤ´ëت©تا________(جî×ضؤ¸´ْ؛إ)،£
a£®¸كخآ¸كر¹
b£®¼سبë´ك»¯¼ء
c£®¼ُةظCO2µؤإ¨¶ب
d£®شِ¼سCOµؤإ¨¶ب
e£®·ضہë³ِ¶¼×أر
(4)زرضھ·´س¦¢ع2CH3OH(g)??CH3OCH3(g)£«H2O(g)ؤ³خآ¶بدآµؤئ½؛â³£تخھ400،£´ثخآ¶بدآ£¬شعأـ±صبفئ÷ضذ¼سبëCH3OH£¬·´س¦µ½ؤ³ت±؟ج²âµأ¸÷×é·ضµؤإ¨¶ببçدآ£؛
خïضت | CH3OH | CH3OCH3 | H2O |
إ¨¶ب/(mol،¤L£1) | 0.44 | 0.6 | 0.6 |
¢ظ±ب½د´ثت±ص،¢ؤو·´س¦ثظآتµؤ´َذ،£؛vص________vؤو(جî،°£¾،±،¢،°£¼،±»ٍ،°£½،±)،£
¢عبô¼سبëCH3OH؛َ£¬¾10 min·´س¦´ïµ½ئ½؛⣬´ثت±c(CH3OH)£½________£»¸أت±¼نؤع·´س¦ثظآتv(CH3OH)£½________،£