��Ŀ����
��9�֣�Ư�����ճ������е�һ�ֳ�����������ɱ������Ư���������Ҫ��ش����⣺
��1��Ư�۵���Ч�ɷ��Ǵ������[Ca(ClO)2]��������ͨ����ʯ�ң���ˮ������������С��1%��������ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ ______________________________��
��2��Ư�۾���Ư����������ΪCa(ClO)2ˮ�������˴����ᣨHClO����д����ˮ�ⷴӦ�����ӷ���ʽ_______________________________��
��3��Ca(ClO)2��Һ�и�����Ũ���ɴ�С��˳����____________________________��
��4�������еĶ�����̼���Կ���ǿƯ�۵�Ư��Ч�����û�ѧ����ʽ��ʾ��ԭ��__________________________________________��
��5��Ư��������ǿ�������ʻ��ʹ�ÿ�����һ���ж����壬д���÷�Ӧ�����ӷ���ʽ________________________________________��
��1��2Cl2+2Ca(OH)2 = CaCl2 +Ca(ClO)2 +2H2O ��2�֣�
��2��ClO-+ H2O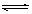 HClO +OH- ��2�֣� ��3��c(ClO-)��c(Ca2+)��c(OH-)��c(H+)��1�֣�
HClO +OH- ��2�֣� ��3��c(ClO-)��c(Ca2+)��c(OH-)��c(H+)��1�֣�
��4��Ca(ClO)2+CO2 + H2O = CaCO3��+2HClO (2�֣���5��ClO- + Cl- + H+ = Cl2��+ H2O ��2�֣�
����
