��Ŀ����
A���������е�����ѧ��ѧʵ�����м��ֳ����IJ���������
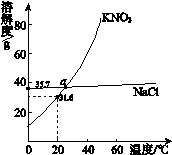
���¶ȼơ� ������ƿ���۵ζ��ܡ����ձ�����������ƿ��
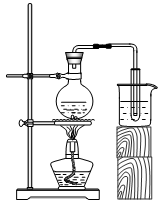
�� ��Ͳ�� �߲���������������
��Ͳ�� �߲���������������
�������Ͼ������ʹ���¶ȵ��ǣ��������¶ȼƣ����������������ţ���
���С�0���̶ȵ������������� �����ţ�
���������ȵ�����������ʱ�������ʯ�����������������������ţ���
ʹ��ʱ�������Ƿ�©ˮ��������������������ţ���
B���������ʵ�����Ũ��Ϊa mol / L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�
����ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ����� ��
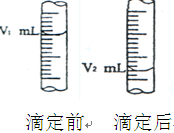
����ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�������c (NaOH)������������ ��
C�������ձ����ڼ�������������ij��Һ50mL��Ӧѡ����ձ��ǣ�����ĸ�ش���������
D����ĥɰ�������Ӳ��������ܷ��Ե�һ�ִ������գ���ʹ������©Һ��©���������������У����Թ� �ڷ�Һ©�� ��ϸ���Լ�ƿ �ܹ���Լ�ƿ �ݼ���ƿ ��ƿ �϶��ò�����ĥɰ�������������������� ������ţ���
���¶ȼơ� ������ƿ���۵ζ��ܡ����ձ�����������ƿ��
��
 ��Ͳ�� �߲���������������
��Ͳ�� �߲����������������������Ͼ������ʹ���¶ȵ��ǣ��������¶ȼƣ����������������ţ���
���С�0���̶ȵ������������� �����ţ�
���������ȵ�����������ʱ�������ʯ�����������������������ţ���
ʹ��ʱ�������Ƿ�©ˮ��������������������ţ���
B���������ʵ�����Ũ��Ϊa mol / L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�
����ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ����� ��

����ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�������c (NaOH)������������ ��
C�������ձ����ڼ�������������ij��Һ50mL��Ӧѡ����ձ��ǣ�����ĸ�ش���������
| A��400mL | B��250mL | C��100mL | D��50 ml |
(17��)A���� �� �ޣ� �� �ۣ� �� �ݣ� �� ��
B�����ñ� ������ϴ�ζ���2��3�� ��a(V2��V1)��Vmol / L���� ��
������ϴ�ζ���2��3�� ��a(V2��V1)��Vmol / L���� ��
C�� C�� D�� ��
B�����ñ�
 ������ϴ�ζ���2��3�� ��a(V2��V1)��Vmol / L���� ��
������ϴ�ζ���2��3�� ��a(V2��V1)��Vmol / L���� ��C�� C�� D�� ��
A��������ƿ���۵ζ��ܡ��� ��Ͳ���������ƻ���ȡ��Һ�IJ������������������������������¶ȣ���Ҫ���ڸ��¶��½���ʵ�飬���������٢ڢۢ��п̶Ȼ���ߣ�����Ͳ��0�̶ȣ�����ƿֻ�б��ߣ��������ȵ������Тܢݢ࣬����ʱ�������ʯ�������Тܢݣ�ʹ��ʱ�������Ƿ�©ˮ���Тڢۡ�B���ζ���������ˮϴ����������װ��Һ��ϴ������������ͼ֪��ȥ��������ΪV2��V1����n(NaOH)=n(HCl)= a(V2��V1), c (NaOH)��a(V2��V1)��V mol / L; C�����ձ����ڼ�������������ij��Һ��Һ�������������ձ��ݻ���һ�룻D����Һ©����������©Һ��ϸ���Լ�ƿ������Լ�ƿ������ƿ����ƿƿ����©������ˢڢۢܢݢ�Ҫĥɰ��
��Ͳ���������ƻ���ȡ��Һ�IJ������������������������������¶ȣ���Ҫ���ڸ��¶��½���ʵ�飬���������٢ڢۢ��п̶Ȼ���ߣ�����Ͳ��0�̶ȣ�����ƿֻ�б��ߣ��������ȵ������Тܢݢ࣬����ʱ�������ʯ�������Тܢݣ�ʹ��ʱ�������Ƿ�©ˮ���Тڢۡ�B���ζ���������ˮϴ����������װ��Һ��ϴ������������ͼ֪��ȥ��������ΪV2��V1����n(NaOH)=n(HCl)= a(V2��V1), c (NaOH)��a(V2��V1)��V mol / L; C�����ձ����ڼ�������������ij��Һ��Һ�������������ձ��ݻ���һ�룻D����Һ©����������©Һ��ϸ���Լ�ƿ������Լ�ƿ������ƿ����ƿƿ����©������ˢڢۢܢݢ�Ҫĥɰ��
 ��Ͳ���������ƻ���ȡ��Һ�IJ������������������������������¶ȣ���Ҫ���ڸ��¶��½���ʵ�飬���������٢ڢۢ��п̶Ȼ���ߣ�����Ͳ��0�̶ȣ�����ƿֻ�б��ߣ��������ȵ������Тܢݢ࣬����ʱ�������ʯ�������Тܢݣ�ʹ��ʱ�������Ƿ�©ˮ���Тڢۡ�B���ζ���������ˮϴ����������װ��Һ��ϴ������������ͼ֪��ȥ��������ΪV2��V1����n(NaOH)=n(HCl)= a(V2��V1), c (NaOH)��a(V2��V1)��V mol / L; C�����ձ����ڼ�������������ij��Һ��Һ�������������ձ��ݻ���һ�룻D����Һ©����������©Һ��ϸ���Լ�ƿ������Լ�ƿ������ƿ����ƿƿ����©������ˢڢۢܢݢ�Ҫĥɰ��
��Ͳ���������ƻ���ȡ��Һ�IJ������������������������������¶ȣ���Ҫ���ڸ��¶��½���ʵ�飬���������٢ڢۢ��п̶Ȼ���ߣ�����Ͳ��0�̶ȣ�����ƿֻ�б��ߣ��������ȵ������Тܢݢ࣬����ʱ�������ʯ�������Тܢݣ�ʹ��ʱ�������Ƿ�©ˮ���Тڢۡ�B���ζ���������ˮϴ����������װ��Һ��ϴ������������ͼ֪��ȥ��������ΪV2��V1����n(NaOH)=n(HCl)= a(V2��V1), c (NaOH)��a(V2��V1)��V mol / L; C�����ձ����ڼ�������������ij��Һ��Һ�������������ձ��ݻ���һ�룻D����Һ©����������©Һ��ϸ���Լ�ƿ������Լ�ƿ������ƿ����ƿƿ����©������ˢڢۢܢݢ�Ҫĥɰ��
��ϰ��ϵ�д�
�����Ŀ