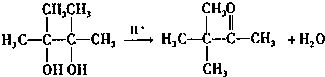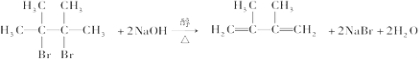��Ŀ����
ij����A�ķ���ʽΪC6H12����֪����������е�̼ԭ�ӹ�ƽ�棬�÷��ӵ�һ��ȡ����ֻ��һ�֣��ش��������⣺��1��A�Ľṹ��ʽΪ______������������A�����������ϣ��������ʵ���һ�������ȼ����������������ȵ��ǣ�����ţ�______��
a��C7H12O2 b��C6H12 c��C6H12O d��C7H14O3
��2��A��Br��CCl4��Һ��Ӧ����B��B��NaOH������Һ���ȿɵõ�D��D����������ԭ�ӣ���д����B�Ʊ�D�Ļ�ѧ��Ӧ����ʽ��______��
��3��B������NaOHˮ��Һ��ȫ��Ӧ�������л���E��
��E���ܷ����ķ�Ӧ�����У�����ţ�______
a��ȡ����Ӧ b����ȥ��Ӧ c���Ӿ۷�Ӧ d����ԭ��Ӧ
��E���Ҷ����Ĺ�ϵ�ǣ�����ţ�______
a��ͬ���칹�� b��ͬһ���� c��ͬϵ�� d��ͬ��������
��4��E���Ҷ�����һ�����������ɷ���ʽΪC8H12O4���л����д���÷�Ӧ�Ļ�ѧ����ʽ______��
��5����֪��
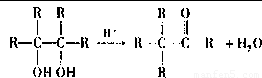 ��-RΪ����������д��E����������������G�Ļ�ѧ��Ӧ����ʽ��______����G������ͬ���ܷ���������Ӧ���ҽṹ�������������칹�干��______�֣�
��-RΪ����������д��E����������������G�Ļ�ѧ��Ӧ����ʽ��______����G������ͬ���ܷ���������Ӧ���ҽṹ�������������칹�干��______�֣�
���𰸡���������1������A�ķ���ʽΪC6H12����֪����������е�̼ԭ�ӹ�ƽ�棬�÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ��CH3��2C=C��CH3��2��
���л���ΪCxHyOz����������Ϊx+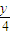 -
- ���Դ˷�����
���Դ˷�����
��2��A��B�����ӳɷ�Ӧ��B��D������ȥ��Ӧ��
��3����BΪ��CH3��2CBrCBr��CH3��2����������NaOHˮ��Һ��ȫ��Ӧ�������л���E��EΪ����
�ڸ���E�Ľṹ��ʽ�жϣ�
��4��EΪ��CH3��2COHCOH��CH3��2��E���Ҷ��ᷢ��������Ӧ��
��5��E�����������·�����ȥ��Ӧ����ͪ����������д��G��ͬ���칹�壮
����⣺��1������A�ķ���ʽΪC6H12����֪����������е�̼ԭ�ӹ�ƽ�棬�÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ��CH3��2C=C��CH3��2��
AΪ��CH3��2C=C��CH3��2��1molA��������Ϊ9mol��1molC7H12O2��������Ϊ7+ -
- =9mol��
=9mol��
1molC6H12��������Ϊ9mol��1molC6H12O��������Ϊ6+3-0.5=8.5��1molC7H14O3��������Ϊ��7+3.5-1.5��mol=9mol��
�ʴ�Ϊ����CH3��2C=C��CH3��2��acd��
��2��A��B�����ӳɷ�Ӧ��B��D������ȥ��Ӧ������B�Ʊ�D�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3����B���������Ƶ�ˮ��Һ����ȡ����Ӧ����2��3-����-2��3-��������2��3-����-2��3-�������ܷ���ȡ����Ӧ����ȥ��Ӧ����ѡab��
��E���Ҷ����ṹ���ƣ��ڷ�����������4��-CH2ԭ���ţ�������ͬϵ���ѡc��
��4��E���Ҷ�����һ�����������ɷ���ʽΪC8H12O4���л���������������ʽ֪��E���Ҷ�����1��1����������Ӧ����Ӧ����ʽΪ��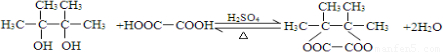 ��
��
�ʴ�Ϊ��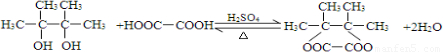 ��
��
��5�����������Ϣ֪��E����������������G�ķ�Ӧ����ʽΪ�� ����G������ͬ���ܷ���������Ӧ˵������ȩ�����ҽṹ�����������������������G��ͬ���칹���У�5-����ȩ��4-����ȩ��3-����ȩ��2-�һ���ȩ��������4�֣�
����G������ͬ���ܷ���������Ӧ˵������ȩ�����ҽṹ�����������������������G��ͬ���칹���У�5-����ȩ��4-����ȩ��3-����ȩ��2-�һ���ȩ��������4�֣�
�ʴ�Ϊ��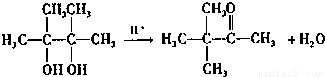 ��4��
��4��
���������⿼���л�����ƶϼ����ʣ�ע�������Ϣ�����ʵĽṹ�ƶϳ�AΪ�����Ĺؼ�����ȷ��ϩ�Ľṹ���Ƴ�A�ǽ�����ͻ�ƿڣ���5��Ϊ�����ѵ㣬��Ŀ�Ѷ��еȣ�
���л���ΪCxHyOz����������Ϊx+
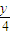 -
- ���Դ˷�����
���Դ˷�������2��A��B�����ӳɷ�Ӧ��B��D������ȥ��Ӧ��
��3����BΪ��CH3��2CBrCBr��CH3��2����������NaOHˮ��Һ��ȫ��Ӧ�������л���E��EΪ����
�ڸ���E�Ľṹ��ʽ�жϣ�
��4��EΪ��CH3��2COHCOH��CH3��2��E���Ҷ��ᷢ��������Ӧ��
��5��E�����������·�����ȥ��Ӧ����ͪ����������д��G��ͬ���칹�壮
����⣺��1������A�ķ���ʽΪC6H12����֪����������е�̼ԭ�ӹ�ƽ�棬�÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ��CH3��2C=C��CH3��2��
AΪ��CH3��2C=C��CH3��2��1molA��������Ϊ9mol��1molC7H12O2��������Ϊ7+
 -
- =9mol��
=9mol��1molC6H12��������Ϊ9mol��1molC6H12O��������Ϊ6+3-0.5=8.5��1molC7H14O3��������Ϊ��7+3.5-1.5��mol=9mol��
�ʴ�Ϊ����CH3��2C=C��CH3��2��acd��
��2��A��B�����ӳɷ�Ӧ��B��D������ȥ��Ӧ������B�Ʊ�D�Ļ�ѧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
����3����B���������Ƶ�ˮ��Һ����ȡ����Ӧ����2��3-����-2��3-��������2��3-����-2��3-�������ܷ���ȡ����Ӧ����ȥ��Ӧ����ѡab��
��E���Ҷ����ṹ���ƣ��ڷ�����������4��-CH2ԭ���ţ�������ͬϵ���ѡc��
��4��E���Ҷ�����һ�����������ɷ���ʽΪC8H12O4���л���������������ʽ֪��E���Ҷ�����1��1����������Ӧ����Ӧ����ʽΪ��
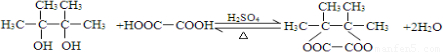 ��
���ʴ�Ϊ��
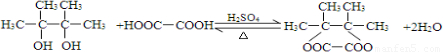 ��
����5�����������Ϣ֪��E����������������G�ķ�Ӧ����ʽΪ��
 ����G������ͬ���ܷ���������Ӧ˵������ȩ�����ҽṹ�����������������������G��ͬ���칹���У�5-����ȩ��4-����ȩ��3-����ȩ��2-�һ���ȩ��������4�֣�
����G������ͬ���ܷ���������Ӧ˵������ȩ�����ҽṹ�����������������������G��ͬ���칹���У�5-����ȩ��4-����ȩ��3-����ȩ��2-�һ���ȩ��������4�֣��ʴ�Ϊ��
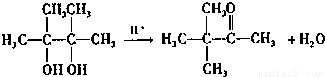 ��4��
��4�����������⿼���л�����ƶϼ����ʣ�ע�������Ϣ�����ʵĽṹ�ƶϳ�AΪ�����Ĺؼ�����ȷ��ϩ�Ľṹ���Ƴ�A�ǽ�����ͻ�ƿڣ���5��Ϊ�����ѵ㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ
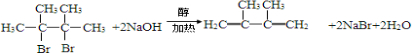
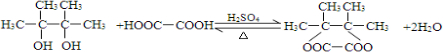
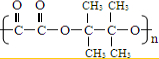
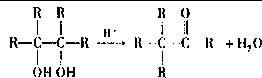 ��-RΪ����������д��E����������������G�Ļ�ѧ��Ӧ����ʽ��
��-RΪ����������д��E����������������G�Ļ�ѧ��Ӧ����ʽ��