��Ŀ����
��֪NO2��NaOH��Һ��Ӧ��2NO2+2NaOH��NaNO2+NaNO3+H2O��NO��NO2��һ����NaOH��Һ���ã�NO2+NO+2NaOH��2NaNO2+H2O������V Lij�ռ���Һ����ȫ����n mol NO2��m mol NO��ɵĻ�����壮
��1�������ռ���Һ�����ʵ���Ũ������Ϊ______��
��2����������Һ��c��NO3-����c��NO2-��=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=______��
��3���ú�n��m�Ĵ���ʽ��ʾ������Һ��NO3-��NO2-����Ũ�ȵı�ֵc��NO3-����c��NO2-��=______��
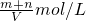 ���ʴ�Ϊ��
���ʴ�Ϊ��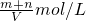 ��
����2����ԭ���������NO2��NO�����ʵ���n��m��
NO2+NO+2NaOH��2NaNO2+H2O��
1 1 2
mmol mmol 2mmol
2NO2+2NaOH��NaNO2+NaNO3+H2O��
2 1 1
��n-m��mol
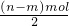
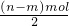
������Һ��c��NO3-����c��NO2-��=
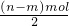 ��[2m+
��[2m+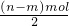 ]=1��9��
]=1��9������n��m=3��2���ʴ�Ϊ��3��2��
��3��ͬһ��Һ�У�c��NO3-����c��NO2-�����������ʵ���֮�ȣ�����c��NO3-����c��NO2-��=
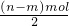 ��[2m+
��[2m+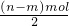 ]=
]=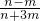 ���ʴ�Ϊ��
���ʴ�Ϊ��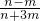 ��
����������1������������������ƵĹ�ϵʽ���㣻
��2�����ݶ���������һ���������������Ʒ�Ӧ�ķ���ʽ�и���������֮��Ĺ�ϵʽ���㣻
��3��ͬһ��Һ�У�c��NO3-����c��NO2-�����������ʵ���֮�ȣ�
���������⿼�����ʵ������йؼ��㣬���ݷ���ʽ�и�������֮��Ĺ�ϵʽ��������ɣ��ѶȲ���

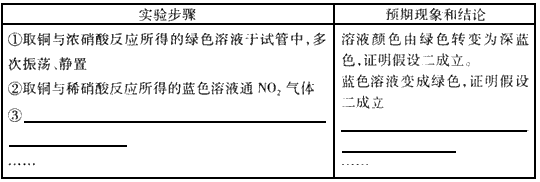
(14�֣���֪�������Һ��ɫΪ��ɫ.ijѧУ��ѧ����С��ͬѧ������ͭ��Ũ���ᡢϡ���ᷴӦ��ʵ���У�����ͭ��Ũ���ᷴӦ����ҺΪ��ɫ���к���ɫ�����������ͭ��ϡ���ᷴӦ����ҺΪ��ɫ,������ɫ����.ΪŪ�巴Ӧ����Һ��ɫ���ֲ����ԭ�����ǽ�����ʵ��̽��.
[ʵ��]����֧�Թ��и�����һС���������ͭƬ���ֱ���˵������ŨHN03��14 mol •
L��1����ϡHN03(4mol . L��1 ), ������պ��NaOH������ס�Թܿڡ���ַ�Ӧ��ͭ����ʣ�ࡣ
(1) Cu��ŨHNO3��Ӧ�����ӷ���ʽ________________________________
(2) ��պ��NaOH������ס�Թܿڵ�ԭ��________________
��֪NO2��NaOH��Һ��Ӧ�У�����NO2�Ļ�ԭ�������������Σ�NO2- )
д����Ӧ�Ļ�ѧ��Ӧʽ________________________,
[�������]����һ����Ϊͭ����Ũ�ȴ���ɵġ�
���������Һ����ɫ����Ϊͭ��Ũ���ᷴӦʱ�����Ķ����������ڹ���Ũ����,���Ϻ���ɫ������ͭ��Һ����һ��,ʹ��Һ����ɫ��
[���ʵ�鷽��,��֤����]
(3) ����ʵ��________(���ܡ����ܡ���֤������һ������,ԭ��________________
(4) ��ͬѧ���ʵ����֤����������������±������ݣ���ʾ:NO3-�ڲ�ͬ�����µĻ�ҧ����ϸ���,��ʱ���Թ۲쵽���������
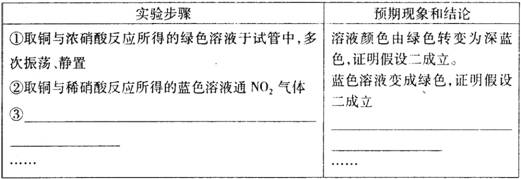
[����������
(5)ͭ��ϡ���ᷴӦ����ҺΪ��ɫ��ԭ��________________