��Ŀ����
��12�֣�ʵ�����ù����ռ�����500mL0.1mol/L��NaOH��Һ��
��1������� g�ռ���塣����Ӧ���� �г�����
��2�����ƹ����У�����Ҫʹ�õ������� ����д���ţ�
��3������ʵ���ʵ����Ҫ�ͣ�2�����г��������жϣ����ʵ�黹ȱ�ٵ������� ��
��4��������ƿ��ȷ����Һ����Ĺ����У����ڼ�������ˮ�������� ��
��5��Ҫ���к�Ũ��Ϊ0.2mol/L���Ϊ10.0mL�����ᣬ��Ҫ�������Ƶ�NaOH��Һ
mL��
��1������� g�ռ���塣����Ӧ���� �г�����
��2�����ƹ����У�����Ҫʹ�õ������� ����д���ţ�
| A���ձ� | B�������� | C��1000mL����ƿ | D��©��E����ͷ�ι�F��ҩ�� |
��4��������ƿ��ȷ����Һ����Ĺ����У����ڼ�������ˮ�������� ��
��5��Ҫ���к�Ũ��Ϊ0.2mol/L���Ϊ10.0mL�����ᣬ��Ҫ�������Ƶ�NaOH��Һ
mL��
��1��2.0 �ձ�
��2��CD
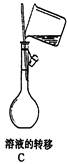
��3����������ƽ ��500mL����ƿ
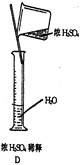
��4���ý�ͷ�ι���μ�ˮ����Һ��İ�Һ��������ƿ�Ŀ̶�������
��5��20.0
��2��CD
��3����������ƽ ��500mL����ƿ
��4���ý�ͷ�ι���μ�ˮ����Һ��İ�Һ��������ƿ�Ŀ̶�������
��5��20.0
��

��ϰ��ϵ�д�
�����Ŀ

 Mg2+ +2OH-
Mg2+ +2OH-
 O2
O2