��Ŀ����
����Ŀ�����������л���ش�������⣬
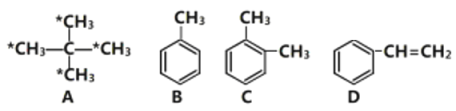
��1���л���A������___________����ע��*����̼ԭ�������������ɵ�ͼ��Ϊ________������Ρ����������Ρ������������Ρ�����E��A��ͬϵ��ұ�A��һ��̼ԭ�ӣ���E��һ�ȴ�����______�֡�
��2����ͬ���������������л�����ȫȼ��ʱ������������_______________(��ṹ��ʽ����
��3���л���B��ʵ������ת����
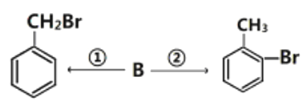
����ת���ķ�Ӧ����Ϊ��_______________����_______________��
��4���л���D�Ĺ���������Ϊ_______________��D��һ�������������ɸ߷��ӻ�����ķ�Ӧ��ѧ����ʽΪ___________________________________________________________��
��5����ͼ�����Ҵ��йص�����ʵ�飬
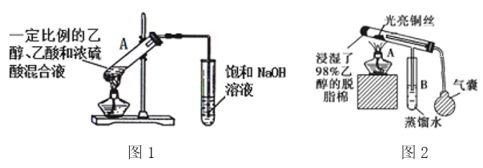
��ͼ1�Թ�A�еķ�Ӧ����ʽΪ______________________________��Ũ�����������_________����ָ��ͼ1���������ԵĴ���_________________________________________��
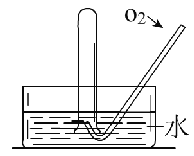
�ڵ�ȼͼ2�оƾ��ƣ�������ѹ���ң���ͼ2װ���й��������ͭ˿���������ɺ������ɺڱ�����������ͭ˿�ɺڱ���ԭ����__________________________________���û�ѧ����ʽ��ʾ����
���𰸡������飨��2,2���������飩����������4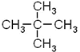 ������������Һ�壨���壩��FeBr3��Fe̼̼˫��
������������Һ�壨���壩��FeBr3��Fe̼̼˫��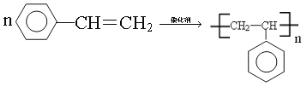 CH3COOH+HOCH2CH3
CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O��������ˮ������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ���CH3CH2OH+CuO
CH3COOCH2CH3+H2O��������ˮ������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ���CH3CH2OH+CuO![]() CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
��������
(1)�л�A�൱�ڼ�������е��ĸ���ԭ�ӱ��ĸ�����ȡ�����ʱ�ע��*����̼ԭ�������������ɵ�ͼ����Ϊ�����壻EΪ���飬�������ֽṹ��CH3(CH2)3-��CH3CH2CH(CH3)-��(CH3)3C-��(CH3)2CHCH2-������һ�ȴ���Ҳ�����֣��ʴ�Ϊ�������飨��2,2���������飩��������������4��(2) ��ͬ����������ȫȼ��ʱ���������˵����������Ԫ�ص�����������ߣ��������л���ķ���ʽ�ֱ�Ϊ��C5H12��C7H8��C8H10��C8H8��ͨ������ʽ�ĶԱȿ�֪A����Ԫ�ص�����������ߣ���Ϊ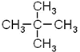 ��(3)����ԭ��ȡ����λ�ÿɵ÷�Ӧ�����ֱ�Ϊ����������������Һ�壨���壩��FeBr3��Fe��(4)�����������ڹ����ţ��ʴ�Ϊ��̼̼˫����
��(3)����ԭ��ȡ����λ�ÿɵ÷�Ӧ�����ֱ�Ϊ����������������Һ�壨���壩��FeBr3��Fe��(4)�����������ڹ����ţ��ʴ�Ϊ��̼̼˫����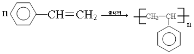 ��(5)ͼ1Ϊ�Ҵ���������Ӧʵ�飬ͼ2Ϊ�Ҵ��Ĵ�����ʵ�飬�ʴ�Ϊ��CH3COOH+HOCH2CH3
��(5)ͼ1Ϊ�Ҵ���������Ӧʵ�飬ͼ2Ϊ�Ҵ��Ĵ�����ʵ�飬�ʴ�Ϊ��CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O����������ˮ��������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ�����CH3CH2OH+CuO
CH3COOCH2CH3+H2O����������ˮ��������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ�����CH3CH2OH+CuO![]() CH3CHO+Cu+H2O��
CH3CHO+Cu+H2O��

 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�