��Ŀ����
(15��)���������Ǵ�����Ⱦ��֮һ��Ϊ���Բⶨ��Χ�����ж�������ĺ�����ij����С��ļס�����λͬѧ�ֱ�����ͬʵ��װ�ú���Һ���ⶨͬһʱ�䣬 ͬһ�ص����(��SO2��N2��O2���壬�����������)��SO2�ĺ�����ʵ��װ������ͼ��Ӧ�Թ���װ�е�ĵ���ϡ��ҺA�� SO2��I2������ӦΪ��S02+I2+2H20=H2SO4+2HI(N2��02����I2�� ���۷�Ӧ)���Իش��������⣺
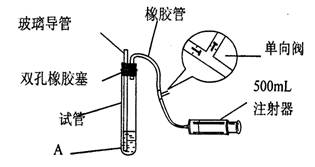
(1)����װ��������ʱ�������Թ���װ��������ˮ(��֤�����ܵ��¶˽���ˮ��)��Ȼ��_____________________(ע�ⷧ�ĵ�����)����֤����װ�õ����������á�
(2)��A��Һ�����ΪVmL��Ũ��Ϊc mol��L-1������Һ����ɫ�պñ�ɫ��ֹͣ��������ʱ�ס�����λͬѧע�����ڳ�����������ֱ�ΪV��mL��V��mL(���е����������ɱ�������)����V��>V�ң���ס��������ⶨ�������õص������S02�����������ʵ�����ӽ�����_______________________(.�ú�c��V��V����V�����ȵĹ�ϵʽ��ʾ)����һλʵ���������ϴ�����ԭ�������_______________________________________________
(3)��������װ�ý��иĽ����������⣬��������װ����Ҫѡ�õ�������___________________�� (ѡ���������ı��)
a���ձ� b���Թ� c����ƿ d������ƿe����Ͳ f�������� g.˫����
(4)��������������ȥ�����еĶ��������ѡ�õ��Լ���___________________��
(1) ������������ע�����������Թ��ڵ��ܿ�������ð��
(2) ���������죬SO2 δ����ˮ�������
���������죬SO2 δ����ˮ�������
(3) beg��ceg��bceg (ֻҪ�𰸺���������)
(4) ����Һ�� (�й������������������𰸾��ɸ���)
����������1�����װ���Dz�©���ģ���������������ע�����������Թ��ڵ��ܿ�������ð�����ݴ˿��Լ��顣
��2����ͬѧ�ó����ʻ�ƫ����˵���������죬SO2 û����ȫ����ˮ������գ����Ӧ������ͬѧ�Ľ��м��㡣���ݷ���ʽ��֪�����ĵĵ��ʵ���0.001Vcmol��ʵ��SO2�������0.0224VcL�����SO2�ĺ����� ��
��
��3��������Ҫ������������Ա�������Ͳ����˴��� beg��ceg��bceg��
��4��SO2����������������ü�Һ���ա�

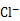 ������VL(�ѻ���Ϊ��״��) A����һ��������ˮ���ټ���a mol FeCl2�ɽ�������Aǡ�ó�ȥ���������ˮ�в���A�����ʵ���Ϊ ��
������VL(�ѻ���Ϊ��״��) A����һ��������ˮ���ټ���a mol FeCl2�ɽ�������Aǡ�ó�ȥ���������ˮ�в���A�����ʵ���Ϊ ��