��Ŀ����
����Ŀ��(1)���л����ֱ������ʲô��������
�ƾ���ˮ_____________��NaCl����ɳ________��KNO3��NaCl_____ ����ˮ��ȡ��________ ��
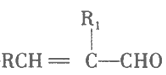
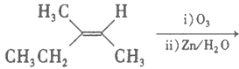
(2)����ĵ���ʽ________����ϩ�Ľṹʽ ________________����Ȳ�ռ乹��_____________����������_________(�л���)̼̼˫�������Ա�________ (�ܻ���)ʹ���������ɫ
(3)������______����ѧʽ����Һ���鱽�ӵĴ��ڣ���������Һ��__________��ȩ�����Ƶ�������ͭ����������___________���ɡ�
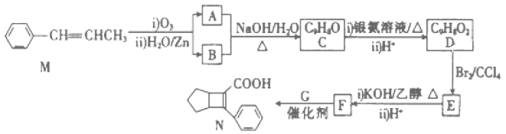
(4)���Ҵ��Ļ�ѧ�����У�����Ӧ�Ķϼ���ʽ�ɸ������£�(�����)
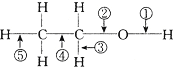
��ȥ��Ӧ��______ ���������Ӧ��_______����������___________�� ��HX��Ӧ��________��
���𰸡����� ���� �ؽᾧ ��ȡ 
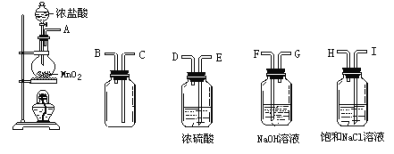
![]() ֱ���� �� ���� FeCl3 ��ɫ ש��ɫ���� �ڢ� �� �٢� ��
ֱ���� �� ���� FeCl3 ��ɫ ש��ɫ���� �ڢ� �� �٢� ��
��������
��1���ƾ���ˮ���ܣ����÷е㲻ͬ���룬���������룬�ʴ�Ϊ����
��ɳ������ˮ������ͨ�����˵ķ������з��룬�ʴ�Ϊ���ˣ�
����ع�����ܽ�����¶ȵ��������������Ȼ��ƹ�����ܽ�����¶ȱ仯Ӱ���С���������¶�Ӱ�첻ͬ�����Բ����ؽᾧ�ķ������Է��룬�ʴ�Ϊ�ؽᾧ��
�岻������ˮ���������л��ܼ�����ѡ�Լ����������Ȼ�̼����ȡ���ٷ�Һ������õ��壬�ʴ�Ϊ��ȡ��
��2��������̼ԭ�Ӻ���ԭ�Ӽ�ͨ�����۵����γɵĿռ���������ṹ������ʽΪ�� ��
��
��ϩ��̼ԭ��֮��ͨ�����Թ��õ��Ӷ��γ�̼̼˫����̼ԭ������ԭ���γ�̼�������ϩ�ĽṹʽΪ��![]() ��
��
��Ȳ�ռ乹��Ϊֱ���ͣ�
��������̼ԭ����̼ԭ��֮���γɵĻ�ѧ���ǽ���̼̼������̼̼˫��֮�������Ļ�ѧ������̼̼˫�������Ա�����ʹ���������ɫ��
(3)������FeCl3��Һ���鱽�ӵĴ��ڣ���������Һ����ɫ��
��ȩ�����Ƶ�������ͭ������������ש��ɫ����������ͭ���ɣ�
(4)�Ҵ�������ȥ��Ӧ������ϩ��ˮ���ڢݼ����ѣ��ʴ�Ϊ:�ڢݣ�
�Ҵ��������Ӧ�����������ټ����ѣ��ʴ�Ϊ:�٣�
�Ҵ�����������O2��Ӧ������ȩ��ˮ���٢ۼ����ѣ��ʴ�Ϊ:�٢ۣ�
�Ҵ���HX��Ӧ����±������ˮ���ڼ�����,�ʴ�Ϊ:�ڡ�

����Ŀ�����������ͬ��ijֲ��Ӫ��Һ�����䷽�ֱ����£�
KCl | K2SO4 | ZnSO4 | |
�� | 0.3 mol��L��1 | 0.2 mol��L��1 | 0.1 mol��L��1 |
�� | 0.1 mol��L��1 | 0.3 mol��L��1 | �� |
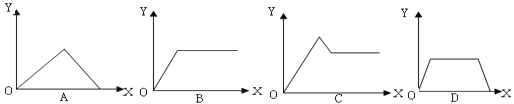
���ж�������Ӫ��Һ�ɷֵķ�������ȷ����(����)
A. K�������ʵ�����ͬ
B. Cl�������ʵ�����ͬ
C. ��ȫ��ͬ
D. SO![]() �����ʵ�����ͬ
�����ʵ�����ͬ