��Ŀ����
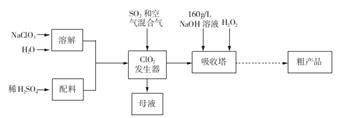
Mn3O4������������������ϵ���Ҫԭ�ϡ������Ṥҵ��β�������Ʊ�����X��Mn3O4�Ĺ����������£�

��ش��������⣺
(1) �����ε��ܽ�ȼ�����ͼ������X�Ļ�ѧʽΪ________��Ϊ��ù���X������KCl��Һ����Ի����Һ��������Ũ�������������������Ӧ________(���������)����ϴ�ӡ����ᄃ�塣
(2) ����X��Ʒ�Ƿ����Ȼ������ʵ�ʵ�������________��
(3) ��ͼ�Ǹ���������������MnSO4��H2Oʱʣ������������¶ȵı仯���ߣ���д��A��B�Ļ�ѧ����ʽ��____________________��
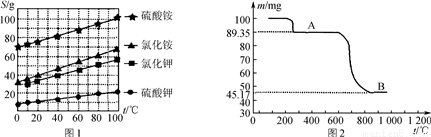
(4) Ϊ�˲ⶨ��Ʒ��Mn3O4�Ĵ��ȣ�ȡ2.500 g��Ʒ��Ũ���Ṳ��(���ʲ������ᷴӦ)��������������ͨ���������۵⻯����Һ�У�ϡ����250 mL������ȡ25.00 mL��0.100 0 mol��L��1 Na2S2O3����Һ�ζ����յ�ʱ������20.00 mL����Һ(2Na2S2O3 ��I2===2NaI��Na2S4O6)��
�� ��Ʒ��Ũ���ᷢ����Ӧ�����ӷ���ʽΪ________��
�� ����Ʒ�Ĵ���Ϊ________��
��ϰ��ϵ�д�
�����Ŀ



 CH3CHBrCH===CH2
CH3CHBrCH===CH2 CH3CHOHCH2CHOCH3CHOHCH2CHO
CH3CHOHCH2CHOCH3CHOHCH2CHO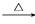 CH3CH===CHCHO��H2O
CH3CH===CHCHO��H2O B��
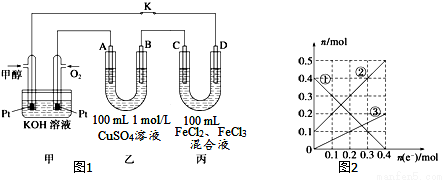
B�� ��ģ��װ����ͼ��ʾ������˵����ȷ����(����)
��ģ��װ����ͼ��ʾ������˵����ȷ����(����)
 ��OH��
��OH��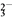 ��H�� D. Fe3����SCN����K��
��H�� D. Fe3����SCN����K�� 3H2O������38 ��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl����ClO2�ֽⱬը��һ���Ʊ��������ƴֲ�Ʒ�Ĺ����������£�
3H2O������38 ��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl����ClO2�ֽⱬը��һ���Ʊ��������ƴֲ�Ʒ�Ĺ����������£�