��Ŀ����
������������Խ������Ϊ��Fe��Al��Ӧ�ù㷺�ġ�������������
(1)Ti��̬ԭ�ӵĵ����Ų�ʽΪ____________��
(2)Ti����B��C��N��O�ȷǽ���Ԫ���γ��ȶ��Ļ����B��C��N�ĵ縺���ɴ�С��˳��Ϊ_____��C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ_____________��
(3)N���⻯�ﳣ���������ԭ����______________��
(4)������ʯ�������ҵ���Ҫ�ɷ�Ϊ����������FeTiO3����FeTiO3��80%�����ᷴӦ������TiOSO4��
SO42-�Ŀռ乹��Ϊ_______�Ρ�������ԭ�Ӳ���_________�ӻ���д��SO42-��һ�ֵȵ�����Ļ�ѧʽ��______��
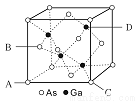
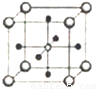
(5)Ti��Ca��O��������γ���ͼ����ṹ��Ti4+λ��������Ķ��㡢Ca2+λ�����������ģ����þ���Ļ�ѧʽΪ________��Ti4+����Χ_____��O2-����ڡ��þ����ı߳�Ϊapm�������ܶ�Ϊ______g/cm3��
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�����ʵ������������Ӧ���õ��Ľ��۾���ȷ����
ѡ�� | ʵ����������� | ���� |
A | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������ã��²���Һ����ɫ | ԭ��Һ����I- |
B | ��ϡHNO3�м��������Fe�ۣ���ַ�Ӧ����K3[Fe(CN)6]��Һ��������ɫ���� | ϡHNO3��Fe����ΪFe2+ |
C | �����£��ⶨ����ʹ�����Һ��pH������pHС�ڴ���PH | ֤����ͬ�����£���ˮ��HCl����̶ȴ���CH3COOH |
D | �����£���pH��ֽ��ã�0.1mol/LNa2SO3��Һ��pHԼΪ10��0.1mol/ LNaHSO3��Һ��pHԼΪ5 | HSO3-���H+��������SO32-��ǿ |
A. A B. B C. C D. D


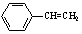
 ���л���X�ļ���ʽΪ
���л���X�ļ���ʽΪ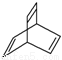 ������˵������ȷ����( )
������˵������ȷ����( )