��Ŀ����
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λ��ͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ����֮���γɵ�������Ϊ
10 �ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣨Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ����
(1)(XY)2����������ԭ������㶼����8���ӽṹ����д����ṹʽ��_____________��(XY)2����W2��������WXY����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ���(KXY)��Һ��ʹ��̪��Һ�Ժ�ɫ���������ӷ���ʽ��ʾ��ԭ��_____________________
(2)��֪��Ȳ��һ�������¿����������ɱ���3CH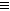 CH
CH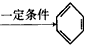 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��__________________��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��__________________��
(3)�����谷��ǿ���ǿ����ˮ��Һ����ˮ�⣬�������ǻ�ȡ�������������ᡣ�����谷���ں������߶���һЩ�̷۳��ҷǷ������̷�����������Ʒ�ĺ��������׳Ƶ����������о����������谷��������������ϸ���н�ϳ����Ӷ��γ�����ʯ��������С�ܣ����������˥�ߣ�������Σ����������д�������谷��ϡ���ᷴӦֱ������������������ӷ���ʽ��_________________________��
(4)��̼�������Ե��ܺġ�����Ⱦ�����ŷ�Ϊ�����ľ���ģʽ������һ�ּ����ǽ�XZ2ת�����л���ʵ��̼ѭ�����磺
2XZ2(g)+2W2Z(1)=X2W4(g)+3Z2(g) ��H= +1411.0 kJ/mol
2XZ2(g) +3W2Z(1)=X2W5ZW(1) +3Z2(g) ��H= +1366.8 kJ/mol
����X2W4ˮ����X2W5ZW��Ӧ���Ȼ�ѧ����ʽΪ______________________
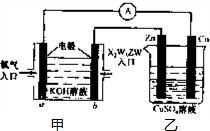
(5)����X2W5ZW ȼ�ϵ�������ͼ��ʾ��װ��
10 �ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣨Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ����
(1)(XY)2����������ԭ������㶼����8���ӽṹ����д����ṹʽ��_____________��(XY)2����W2��������WXY����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ���(KXY)��Һ��ʹ��̪��Һ�Ժ�ɫ���������ӷ���ʽ��ʾ��ԭ��_____________________
(2)��֪��Ȳ��һ�������¿����������ɱ���3CH
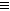 CH
CH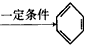 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��__________________��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��__________________��(3)�����谷��ǿ���ǿ����ˮ��Һ����ˮ�⣬�������ǻ�ȡ�������������ᡣ�����谷���ں������߶���һЩ�̷۳��ҷǷ������̷�����������Ʒ�ĺ��������׳Ƶ����������о����������谷��������������ϸ���н�ϳ����Ӷ��γ�����ʯ��������С�ܣ����������˥�ߣ�������Σ����������д�������谷��ϡ���ᷴӦֱ������������������ӷ���ʽ��_________________________��
(4)��̼�������Ե��ܺġ�����Ⱦ�����ŷ�Ϊ�����ľ���ģʽ������һ�ּ����ǽ�XZ2ת�����л���ʵ��̼ѭ�����磺
2XZ2(g)+2W2Z(1)=X2W4(g)+3Z2(g) ��H= +1411.0 kJ/mol
2XZ2(g) +3W2Z(1)=X2W5ZW(1) +3Z2(g) ��H= +1366.8 kJ/mol
����X2W4ˮ����X2W5ZW��Ӧ���Ȼ�ѧ����ʽΪ______________________
(5)����X2W5ZW ȼ�ϵ�������ͼ��ʾ��װ��
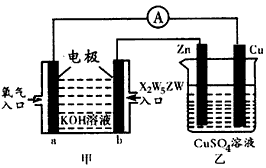
�ٸ�װ����Cu��Ϊ________����
��д��b���ĵ缫��Ӧʽ��_______________��
�۵�ͭƬ�������仯12.8 gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ___________L���ס�����װ���е������ҺpH�ı仯Ϊ��_________����_________ �������� ��С�����䡱��
��д��b���ĵ缫��Ӧʽ��_______________��
�۵�ͭƬ�������仯12.8 gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ___________L���ס�����װ���е������ҺpH�ı仯Ϊ��_________����_________ �������� ��С�����䡱��
(1)N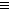 C-C
C-C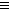 N��CN- +H2O
N��CN- +H2O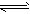 HCN+ OH-
HCN+ OH-
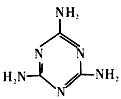
(2)
(3)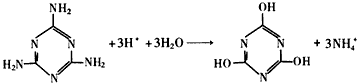
(4)C2H4(g) +H2O(1)=C2H5OH(1) ��H= -44.2 kJ/mol
(5)��������C2H5OH+16OH- -12e- =2CO32-+11H2O����2.24����������
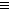 C-C
C-C N��CN- +H2O
N��CN- +H2O HCN+ OH-
HCN+ OH- (2)

(3)
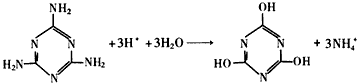
(4)C2H4(g) +H2O(1)=C2H5OH(1) ��H= -44.2 kJ/mol
(5)��������C2H5OH+16OH- -12e- =2CO32-+11H2O����2.24����������

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
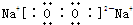

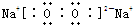
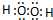
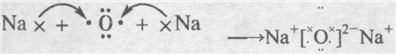

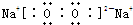
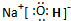
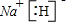
 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��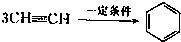 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��