��Ŀ����
A��B��C��D��E����Ԫ�أ�����ԭ�ӵĺ˵�������ε����Ҿ�С��18��AԪ��ԭ�Ӻ���ֻ��1�����ӣ�C�ǵؿ��к�������Ԫ�أ�B��C���γ����ֻ�����BC��BC2��B���������������۾���ֵ��ȣ�BC�ж���BC2���������DԪ�ص�����������������Ӳ���������֮һ��E��������Arԭ����ͬ�ĵ��Ӳ�ṹ��
��1��д��A��C����Ԫ�صķ��ţ�A �� C ��
��2��д��Dԭ�Ӻ�E�����ӵĽṹʾ��ͼ��Dԭ�� ��E������ ��
��3����A��B��C��D����Ԫ����ɵij���������Ļ�ѧʽΪ ��
����뷽��ʽΪ ��
��4��д��A��C��D����Ԫ����ɵij����������ϡ��Һ�������º�E�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ ��
��1��д��A��C����Ԫ�صķ��ţ�A �� C ��
��2��д��Dԭ�Ӻ�E�����ӵĽṹʾ��ͼ��Dԭ�� ��E������ ��
��3����A��B��C��D����Ԫ����ɵij���������Ļ�ѧʽΪ ��
����뷽��ʽΪ ��
��4��д��A��C��D����Ԫ����ɵij����������ϡ��Һ�������º�E�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ ��
��1��H�� O��
��2��
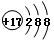
��3��NaHCO3 �� NaHCO3 = Na+ + HCO3����
��4��Cl2 + 2NaOH =" NaCl" + NaClO +H2O��
��2��

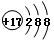
��3��NaHCO3 �� NaHCO3 = Na+ + HCO3����
��4��Cl2 + 2NaOH =" NaCl" + NaClO +H2O��
���������AԪ��ԭ�Ӻ���ֻ��1�����ӣ�����ΪHԪ�أ�C�ǵؿ��к�������Ԫ�أ�ΪOԪ�أ�B��C���γ����ֻ�����BC��BC2��B���������������۾���ֵ��ȣ�BC�ж���BC2�������������BΪ̼Ԫ�أ�DԪ�ص�����������������Ӳ���������֮һ��Ϊ��Ԫ�أ�E��������Arԭ����ͬ�ĵ��Ӳ�ṹ������Ϊ��Ԫ�ء���3����A��B��C��D����Ԫ����ɵij���������Ļ�ѧʽΪNaHCO3��HCO3��Ϊ��Ԫ�������ʽ�������ܲ���4�������ͼ���绯��Ӧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

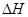 = ������������ ( ע���������浥�ʾ�Ϊ���ȶ�����)��
= ������������ ( ע���������浥�ʾ�Ϊ���ȶ�����)��