��Ŀ����
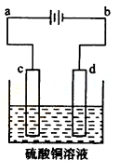
��ͼ��ʾ��U�ι���ʢ��100mL����Һ����Ҫ��ش��������⣺

��1����K2 ���ϲ�K1������ʢ��ҺΪCuSO4��Һ����AΪ ���� A���ĵ缫��ӦʽΪ ������ʢ��ҺΪKCl��Һ����B���ĵ缫��ӦʽΪ
��2����K1���ϲ�K2������ʢ��ҺΪ���з�̪��NaCl��Һ����
��A�缫�����ɹ۲쵽�������� ��Na+���� ������A��B��
��B�缫�ϵĵ缫��ӦʽΪ ��
�ܷ�Ӧ��ѧ����ʽ�� ��
�۷�Ӧһ��ʱ����K2,��������Һ������仯��������ܽ⣬B������������������״����Ϊ11.2mL������Һ��ֻ�ϣ���Һ��pHԼΪ ����Ҫʹ�������Һ�ָ���ԭ״̬������U�ι��ڼ����ͨ��һ������ ��[ pH=-lgc(H+) ]

��1����K2 ���ϲ�K1������ʢ��ҺΪCuSO4��Һ����AΪ ���� A���ĵ缫��ӦʽΪ ������ʢ��ҺΪKCl��Һ����B���ĵ缫��ӦʽΪ
��2����K1���ϲ�K2������ʢ��ҺΪ���з�̪��NaCl��Һ����
��A�缫�����ɹ۲쵽�������� ��Na+���� ������A��B��
��B�缫�ϵĵ缫��ӦʽΪ ��
�ܷ�Ӧ��ѧ����ʽ�� ��
�۷�Ӧһ��ʱ����K2,��������Һ������仯��������ܽ⣬B������������������״����Ϊ11.2mL������Һ��ֻ�ϣ���Һ��pHԼΪ ����Ҫʹ�������Һ�ָ���ԭ״̬������U�ι��ڼ����ͨ��һ������ ��[ pH=-lgc(H+) ]
��1���ٸ���Cu2++2e-=Cu��O2+2H2O+4e-==4OH-
��2���ٸ�װ���ǵ��أ�̼����������п�������������ʱ��п���������ӷŵ�����������ͬʱ�缫�����������������ӵ�����Һ�ʼ��ԣ������̪����Һ��죻�ʴ�Ϊ��������ɫ���ݣ��缫������Һ��죻��A��
�ڵ��ʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ��Һ�������������ƣ����Ե�ط�ӦʽΪ2NaCl+2H2O 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
��12��HCl
��2���ٸ�װ���ǵ��أ�̼����������п�������������ʱ��п���������ӷŵ�����������ͬʱ�缫�����������������ӵ�����Һ�ʼ��ԣ������̪����Һ��죻�ʴ�Ϊ��������ɫ���ݣ��缫������Һ��죻��A��
�ڵ��ʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ��Һ�������������ƣ����Ե�ط�ӦʽΪ2NaCl+2H2O
 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2������12��HCl
�������������1���ٸ�װ����ԭ��أ�п��������̼��������������ͭ���ӵõ�������ͭ����������Ӧ���缫��ӦʽΪCu2++2e-=Cu����ʢ��ҺΪKCl��Һ��Ϊ������ʴ������B���ĵ缫��ӦʽO2+2H2O+4e-==4OH-��
��2����K1���ϲ�K2������ʢ��ҺΪ���з�̪��NaCl��Һ���ٸ�װ���ǵ��أ�̼����������п�������������ʱ��п���������ӷŵ�����������ͬʱ�缫�����������������ӵ�����Һ�ʼ��ԣ������̪����Һ��죻��Һ�е�Na+�������ƶ�������A�缫�ƶ���
�ڵ��ʱ������B�������ӷŵ�����������2Cl��2e-==Cl2���������������ӷŵ�����������ͬʱ��Һ�������������ƣ����Ե�ط�ӦʽΪ2NaCl+2H2O
 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2�������ɢڿ�֪��B����������״����Ϊ11.2mLCl2������0.001molNaOH����c(OH-)=0.01mol/L��c(H+)=Kw/c(OH-)=10-14/0.01mol/L=10-12 mol/L����pH=12�������ݳ�������ֱ���H2��Cl2����Ҫʹ�������Һ�ָ���ԭ״̬������U�ι���ͨ��һ������HCl���塣

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
 KIO3��3H2��������˵����ȷ���ǣ� ��
KIO3��3H2��������˵����ȷ���ǣ� ��