��Ŀ����
��һƿ���������Լ����ֱ���������(2Na2SO3+O2�T�T2Na2SO4)���������������ƣ�Ϊ�˲ⶨ�������Ƶĺ�����ͨ�����и���ʵ���������һ������ȡ����a g��һ����ֽb g
�ڶ���������Ʒ�ܽ���ˮ
������������Һ�м������A��Һ�����������ữ��
���IJ������˺�ϴ�ӳ���
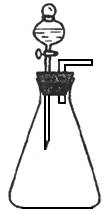
���岽�������ֽ�ͳ���
��������������ֽ�ͳ�����������Ϊc g
�Իش�
��1���ڶ����������õ���������________��
��2�����������������õ���A��________��Һ��A��ҺҪ�ữ��Ŀ����________������A��Һ������ԭ����________________������A�Ƿ�����ķ�����________________��
��3�����IJ�����Ҫϴ�ӳ�����ԭ����________��ϴ�ӳ����ķ�����________________��
��4�������������Ƶ�������������ʽΪ________________��
��5�����ڶ�����������������Һ������ʹ��õĺ�����ʵ�ʵ�________��ƫ��/ƫ�ͣ��������岽��������ֽ�ͳ���δ��ɣ�ʹ��ú�����ʵ�ʵ�________��ƫ��/ƫ�ߣ���
������
| ����ʵ�����Ƕ�������������ɵ���Ŀ������Na2SO3��Na2SO4�����ķ����������������Ͽ������֣���ʹ ��ʹ ���⣬������Ŀ�����������в�����ʧ�������ijɷ֣�Ҳ��Ҫ���ӱ������ijɷ֡��ڼ�������BaCl2�Ƿ����ʱҪ�ر�ע�⡣ �𰸣���1���ձ��������� ��2��BaCl2,��ȥNa2SO3,ʹ ��3����ȥ����,����������ò�����������������ˮ���պý�û����Ϊֹ����ˮ���£��ظ����ϲ���2��3�Ρ� ��4�� ��5��ƫ��,ƫ��
|
