��Ŀ����
(14��)
С��1:����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹ������Ȼ�̼��Һ��ɫ��1 mol A��1 mol NaHCO3����ȫ��Ӧ����A�Ľṹ���� ��
д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ ��
��
С��2:������B����C��H��O����Ԫ�أ���Է�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.3%��B��Cu�Ĵ������±�����������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ�� ������B�ĺ˴Ź������ף�NMR������� �����շ壬��ֵ����Ϊ ��
С��3:D����A��B��Ӧ���ɵ�������Ӧ��Ӧ�Ļ�ѧ����ʽ��______________
С��1:����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹ������Ȼ�̼��Һ��ɫ��1 mol A��1 mol NaHCO3����ȫ��Ӧ����A�Ľṹ���� ��
д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ ��
��
С��2:������B����C��H��O����Ԫ�أ���Է�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.3%��B��Cu�Ĵ������±�����������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ�� ������B�ĺ˴Ź������ף�NMR������� �����շ壬��ֵ����Ϊ ��
С��3:D����A��B��Ӧ���ɵ�������Ӧ��Ӧ�Ļ�ѧ����ʽ��______________
��1:
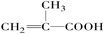 CH3��CH��CH��COOH��CH2��CH��CH2��COOH
CH3��CH��CH��COOH��CH2��CH��CH2��COOH��2:CH3CH2CH2OH 4 3��2��2��1
��3: CH2=C(CH3)COOH��CH3CH2CH2OH
 CH2=C(CH30COOCH2CH2CH3��H2O
CH2=C(CH30COOCH2CH2CH3��H2OС��1:A����ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼�����ͼ�������ΪA��1 mol NaHCO3����ȫ��Ӧ������A�к���1���Ȼ���A�Ǿ���֧���Ļ��������A�Ľṹ��ʽΪ
 ��ͨ���ı�̼̼˫����λ�ü���д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ���ֱ�ΪCH3��CH��CH��COOH��CH2��CH��CH2��COOH��
��ͨ���ı�̼̼˫����λ�ü���д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ���ֱ�ΪCH3��CH��CH��COOH��CH2��CH��CH2��COOH��С��2:̼����������Ϊ60%����̼ԭ������
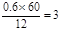 �������������Ϊ13.3%������ԭ������
�������������Ϊ13.3%������ԭ������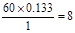 ��������ԭ������
��������ԭ������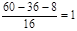 ����˷���ʽΪC3H8O��B�����������ܷ���������Ӧ������B�Ľṹ��ʽΪCH3CH2CH2OH��������к���4����ԭ�ӣ�����֮��Ϊ3��2��2��1��
����˷���ʽΪC3H8O��B�����������ܷ���������Ӧ������B�Ľṹ��ʽΪCH3CH2CH2OH��������к���4����ԭ�ӣ�����֮��Ϊ3��2��2��1��С��3:�������Ϸ�����֪A��B�ķ���������Ӧ�ķ���ʽΪ
CH2=C(CH3)COOH��CH3CH2CH2OH
 CH2=C(CH30COOCH2CH2CH3��H2O��
CH2=C(CH30COOCH2CH2CH3��H2O��
��ϰ��ϵ�д�
�����Ŀ