��Ŀ����
���������ڴ�����SO2��NOx (NO��NO2)�ĺ����������ߣ���ת��ΪH2SO4��HNO3(��Ҫ�� H2SO4)����ˮ������γɵġ�Ϊ��ֹ��Ⱦ�����᳧�ð�ˮ������SO2�����ӷ���ʽΪ_______�����᳧�����ռ���Һ�����յ����������NO2���ռ���Һ��������NaNO2�� NaNO3�����ӷ���ʽΪ____��Ҳ���ð��Ĵ���ԭ���ѵ����������ɵ�������ԭ1�����NOx��Ҫ___���������
2NH3��H2O+SO2=2NH4++SO32-+H2O ��2NO2+2OH-=NO2-+NO3-+H2O ��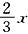

��ϰ��ϵ�д�
�����Ŀ