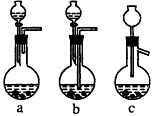��Ŀ����
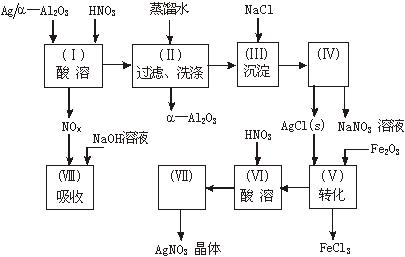
Ag/��Al2O3��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã���Al2O3�������Ҳ��������ᣬ�ô����Ļ���ʵ������ͼ��ʾ�����е�ת����ӦΪ��6AgCl+Fe2O3====3Ag2O+2FeCl3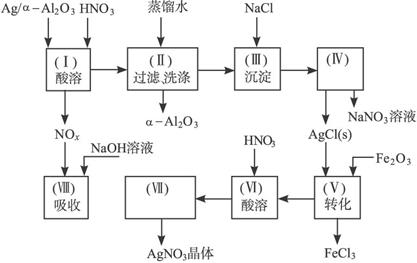
ͼ15-18
�Ķ�����ʵ�����̣����������գ�
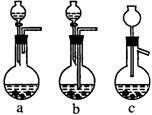
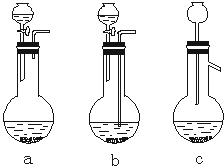
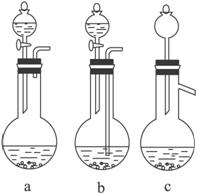
��1��Ag/��Al2O3�����ܽ�Ӧ��ѡ��װ��____________________________��ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽΪ_________________________________��
��3��ʵ��������������貣������Ϊ_________________����д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ��________��
a.���� b.���� c.���� d.���� e.��ȴ�ᾧ
��5����֪��NO+NO2+2NaOH====2NaNO2+H2O��
2NO2+2NaOH====NaNO3+NaNO2+H2O��
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ________________��
a.x��1.5 b.x=1.2 c.x��1.5
��6����֪Ag/��Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ_____��__________��
������������һ���ۺ��Խ�ǿ����Ŀ������ҪŪ��ʵ�����̵�Ŀ�ĺͷ�Ӧԭ����Ȼ�������ȷ��𡣣�1����Ag����HNO3��Al2O3���ܣ���Ag����HNO3ʱ��ų�NOx��Ӧѡװ��a����2����������ˮ����Cl-���������Ag++Cl-====AgCl������3��ʵ������������ǹ��˳�AgCl������������˲��������������Ҫ����Ϊ�ձ�����������©����ע�⿴��Ҫ�ش�������������4��Ҫ��AgNO3��Һ�л��AgNO3���壬�ر�ע�ⲻ�����ա���AgNO3�ֽ⡣��5����������������ʽ������NO���ܱ��������ն�NO2�ɱ��������ա���ô������屻��ȫ����ʱ��x��1.5����6��Ҫ����Ag�Ļ����ʱ���֪����������������������Ag�������������Ag������������Ҫ֪���������AgNO3��������
�𰸣���1��a ��2��Ag++Cl-====AgCl�� (3)©�����ձ��������� ��4��bed ��5��c
��6������������ AgNO3������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�