��Ŀ����
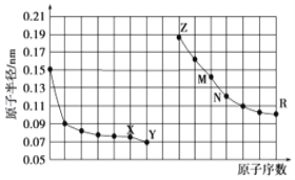
����Ŀ����ͼ��Na��Cu��Si��H��C��N��Ԫ�ص��ʵ��۵�ߵ�˳������c��d�����Ⱥ͵�������塣
��1����д����ͼ��e���ʶ�ӦԪ��ԭ�ӵĵ����Ų�ʽ��________��
��2��a��b��f��Ӧ��Ԫ����ԭ�Ӹ�����1��1��1�γɵķ�������������������֮��Ϊ___��
��3��ͼ��d���ʵľ���ѻ���ʽ�����ǣ�____��
��4��a��bԪ���γɵ�10�������Է���X�Ŀռ乹��Ϊ_____����X����ˮ�����Һ���뺬dԪ�ظ����ӵ���Һ�������������ɵĺ�dԪ�����ӵĻ�ѧʽΪ___������X��d�ĸ�����֮����___����ϡ�
��5����������������Ԫ���е�һ��Ԫ���γɵĺ�����Ľṹ��![]() �����Ҫ˵��������������ˮ��ԭ��_____________��
�����Ҫ˵��������������ˮ��ԭ��_____________��
���𰸡�1s22s22p63s23p2��[Ne]3s23p2 1:1 �����������ܶѻ� ������ [Cu(NH3)4]2�� ��λ HNO3�Ǽ��Է��ӣ�������(���Ե�)ˮ�У�HNO3�����е�H��O����ˮ�����е�O��H֮���γ������
��������
Na��Cu��Si��H��C��N��Ԫ�ص����У�Na��CuΪ�������壬�����Ⱥ͵�������壬C��Si�ĵ���Ϊԭ�Ӿ��壬��C���ʵ��۷е����Siԭ�Ӿ�����۷е㣬H��N��Ӧ�ĵ���Ϊ���Ӿ��壬�����������۵���ͣ���ͼ�۵�ĸߵ�˳���֪aΪH��bΪN��cΪNa��dΪCu��eΪSi��fΪC����϶�Ӧ���ʡ�������Ľṹ�������Լ�Ԫ�������ɵĵݱ���ɽ����⡣
�ɷ�����֪��aΪH��bΪN��cΪNa��dΪCu��eΪSi��fΪC��
��1��eΪSi���䵥�ʶ�ӦԪ��ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p2��[Ne]3s23p2���ʴ�Ϊ��1s22s22p63s23p2��[Ne]3s23p2��
��2������a��b��f��Ӧ��Ԫ����ԭ�Ӹ�����1��1��1�γɵķ���ΪHCN���ṹʽΪH-C��N��������к���1��C-H������1��-C��N��������1��������2��������������H-C��N�к���2��������2����������������������֮��Ϊ1:1���ʴ�Ϊ��1:1��
��3��Cu���ʵľ���ѻ���ʽ�����������������ܶѻ����ʴ�Ϊ�������������ܶѻ���
��4��a��bԪ���γɵ�10�������Է���XΪNH3�����������м۲���ӶԸ���Ϊ=3+![]() ����5-3��1��=4�����Ե�ԭ���ӻ���ʽΪsp3����Ϊ����һ���µ��Ӷԣ��������Ŀռ乹��Ϊ�����Σ���NH3����ˮ�����Һ���뵽��CuԪ�ظ����ӵ���Һ����������Cu2+�ṩ�չ�������������ṩ�¶Ե��ӣ��ṩ��λ���γ��İ���ͭ�����ӣ��仯ѧʽΪ��[Cu(NH3)4]2�����ʴ�Ϊ�������Σ�[Cu(NH3)4]2������λ��
����5-3��1��=4�����Ե�ԭ���ӻ���ʽΪsp3����Ϊ����һ���µ��Ӷԣ��������Ŀռ乹��Ϊ�����Σ���NH3����ˮ�����Һ���뵽��CuԪ�ظ����ӵ���Һ����������Cu2+�ṩ�չ�������������ṩ�¶Ե��ӣ��ṩ��λ���γ��İ���ͭ�����ӣ��仯ѧʽΪ��[Cu(NH3)4]2�����ʴ�Ϊ�������Σ�[Cu(NH3)4]2������λ��
��5�����ݺ�����Ľṹ��֪����ΪHNO3��HNO3�Ǽ��Է��ӣ������ڼ��Ե�ˮ�в���HNO3�����е�H��O����ˮ�����е�O��H֮���γ������������ˮ���ʴ�Ϊ��HNO3�Ǽ��Է��ӣ�������(���Ե�)ˮ�У�HNO3�����е�H��O����ˮ�����е�O��H֮���γ������

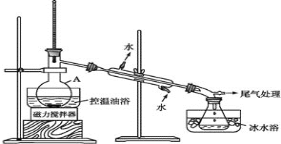
����Ŀ��ʵ�������ܶ�Ϊ1.25 g/mL����������Ϊ36.5%��Ũ��������240 mL 0.1 mol/L�����ᣬ��ش��������⣺
(1)Ũ��������ʵ���Ũ��Ϊ_________��
(2)���� 240 mL 0.1mol/L�����ᡣ
Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ��/mL |
______ | ______ |
(3)����ʱ������ȷ�IJ���˳����(��ĸ��ʾ��ÿ����ĸֻ����һ�� )_________
A. ��30 mLˮϴ���ձ��ڱںͲ�����23�Σ�ϴ��Һ��ע������ƿ����
B. ����Ͳ��ȷ��ȡ�����Ũ���������������ձ��У��ټ�������ˮ(Լ30 mL)���ò���������������ʹ���Ͼ���
C. ������ȴ�������ز�����ע������ƿ��
D. ������ƿ�ǽ�����ҡ��
E. ���ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�������
F. ����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���12 cm��
(4)��ʵ�������������������������Һ�����ʵ���Ũ���к�Ӱ��(����ƫ��������ƫ��������������)?
������ϡ��������ձ�δϴ��_________��
������ƿ��ԭ����������ˮ_________��
�۶���ʱ�۲�Һ�温��_________��
����Ŀ���±���Ԫ�����ڱ���һ���֣��û�ѧ����ش��������⣺
��A | ��A | ��A | ��A | ��A | ��A | ��A | |
�� | �� | �� | �� | ||||
�� | �� | �� | �� | �� | �� |
��1���۵�Ԫ�ط���Ϊ___��Ԫ�آ�����γɻ�����ĵ���ʽΪ___��
��2���Ƚ�Ԫ�آٺ͢�ԭ�Ӱ뾶��С����___������>������<������
��3��Ԫ�آ����ӽṹʾ��ͼΪ___��
��4��Ԫ�آٺ͢��γɵĻ������к��еĻ�ѧ��Ϊ___��
��5��Ԫ�آڡ��ݵ�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽΪ___��
��6����ҵ��ұ��Ԫ�آĵ��ʵĻ�ѧ����ʽΪ___��