��Ŀ����
����Ŀ��ij������������Al��(NH4)2SO4��MgCl2��FeCl2��AlCl3��KCl�е����ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ(������������ѻ���ɱ�״���µ����)��
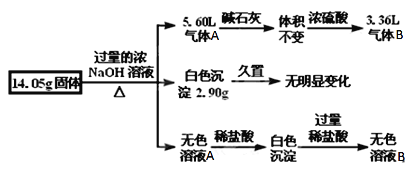
��1������B�ijɷ���_________��������B�ijɷ��Ʋ�һ�����е����ʵ�����Ϊ________g��
��2���ù���������_____����С�����û�С�����ȷ������KCl��������__________________���������һ�ּķ�������ù������Ƿ���KCl________________________________��
��3����ȡ14.05g������������1L 0.4mol/LNaHSO4��Һ�У�������Һ�����������ʵ���Ũ��֮��Ϊ________��
���𰸡�H2 2.7g û�� �������㣬�����к���Al��(NH4)2SO4��MgCl2�����������պ�Ϊ14.05g�����Բ�����KCl ȡ�ù����ھƾ��ƻ��������գ�����ɫ�ܲ����۲�����Ƿ�����ɫ����û����ɫ����û��KCl 9:2��2:9
��������
��������ͼ��֪���ù������������������Ʒ�Ӧ��������壬���������Ʒ�Ӧ��������Ĺ��������Al������泥�������ͨ��Ũ���ᣬ�������2.24L��˵����������AΪ���������Ϊ2.24L����3.36L����BΪ������˵�������к���Al����������ͨ��������������㣻��������������ƺ������ɫ�����������ޱ仯��˵�������Ȼ�������һ�������Ȼ�þ���õ�����Һ�м���ϡ�����Ȳ���������������ܽ⣬˵����ʱ��Һ�к���ƫ��������ӣ���Ϊ������һ����Al�����Բ�һ�������Ȼ������������Ϸ���������������һ������Al������李��Ȼ�þ���Դ˽��
��������ͼ��֪���ù������������������Ʒ�Ӧ��������壬���������Ʒ�Ӧ��������Ĺ��������Al������泥�������ͨ��Ũ���ᣬ�������2.24L��˵����������AΪ���������Ϊ2.24L����3.36L����BΪ������˵�������к���Al����������ͨ��������������㣻��������������ƺ������ɫ�����������ޱ仯��˵�������Ȼ�������һ�������Ȼ�þ���õ�����Һ�м���ϡ�����Ȳ���������������ܽ⣬˵����ʱ��Һ�к���ƫ��������ӣ���Ϊ������һ����Al�����Բ�һ�������Ȼ������������Ϸ���������������һ������Al������李��Ȼ�þ��
��1��ͨ�����Ϸ���������B�ijɷ���H2��������B�ijɷ��Ʋ�һ�����е�����ΪAl��Al������������Һ��Ӧ����H2������2.24L H2��ҪAl������Ϊ![]() ��
��![]() ��27g/mol=2.7g��
��27g/mol=2.7g��
�ʴ�Ϊ��H2 ��2.7��
��2��ͨ�����Ϸ������ù���������һ������Al������李��Ȼ�þ��
���к�Al������Ϊ2.7g��
������淋�����Ϊ��![]() ��132g/mol=6.6g��
��132g/mol=6.6g��
���Ȼ�þ������Ϊ��![]() ��95g/mol=4.75g��
��95g/mol=4.75g��
��Ϊ2.7g+6.6g+4.75g=14.05g�����Ի����ֻ��Al������李��Ȼ�þ��ɣ�����KCl��
��ͨ����ɫ��Ӧ����K+��ȷ���ù������Ƿ���KCl��
�ʴ�Ϊ��û�У��������㣬�����к���Al��(NH4)2SO4��MgCl2�����������պ�Ϊ14.05g�����Բ�����KCl��ȡ�ù����ھƾ��ƻ��������գ�����ɫ�ܲ����۲�����Ƿ�����ɫ����û����ɫ����û��KCl��
��3��1L 0.4mol/LNaHSO4��Һ�к���0.4mol H+��0.4mol SO42-�������Al��(NH4)2SO4��MgCl2����Ϻ��еĵ�������ΪSO42-��Cl-�����к�SO42-Ϊ0.4mol+0.05mol=0.45mol����Cl-Ϊ0.05mol��2=0.1mol��SO42-��Cl-���ʵ���Ũ��֮��=���ߵ����ʵ���֮��=0.45:0.1=9:2��
�ʴ�Ϊ��9:2��2:9��

����Ŀ��Ϊ��֤������Cl2 > Fe3+ > SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��

ʵ����̣�
���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
����a���μ�һ������Ũ���ᣬ��A���ȡ�
��B����Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��������b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
�������ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
���������Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1�����̢��Ŀ����_______________________________________________��
��2�����н������Һ��ѧʽΪ_________________________��
��3��A�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��4�����̢��м�������Fe3+ ��Fe2+���Լ��ֱ�Ϊ___________��__________��
ȷ����Fe3+ ��Fe2+����ֱ���_______________��____________________��
��5�����̢�������B��Һ���Ƿ���SO42���IJ�����_____________________��
��6���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤�������ԣ�
���̢� B��Һ�к��е����� | ���̢� B��Һ�к��е����� | |
�� | ��Fe3+��Fe2+ | ��SO |
�� | ����Fe3+����Fe2+ | ��SO |
�� | ��Fe3+��Fe2+ | ��Fe2+ |
Cl2 > Fe3+ > SO2����________����ס����ҡ�����������