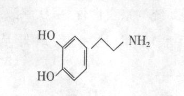
��Ŀ����
��16�֣���֪��һ�������£�ϩ���ɷ�������������Ӧ��
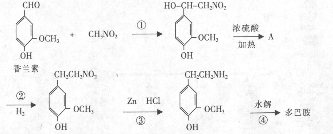
ij�߷��ӻ����C5H8��n�ܹ��������·�Ӧ��
����������Ϣ������������
��1��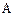 ��������__________��
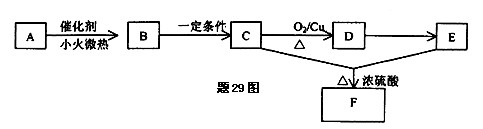
������Ϊ��__________��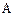 �ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________��
�ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________��
��2��д�� ת��Ϊ
ת��Ϊ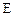 �Ļ�ѧ����ʽ____________________��
�Ļ�ѧ����ʽ____________________��
��3��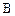 ��ͬ���칹��
��ͬ���칹��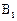 ������ֻ��һ���������ܷ���ˮ�ⷴӦ����
������ֻ��һ���������ܷ���ˮ�ⷴӦ����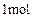
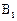 ������������Һ��Ӧ������ܵ�
������������Һ��Ӧ������ܵ�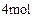
 ����
����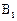 ���п��ܵĽṹ��ʽΪ��__________��
���п��ܵĽṹ��ʽΪ��__________��

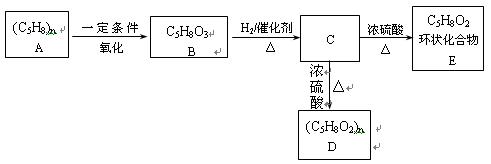
ij�߷��ӻ����C5H8��n�ܹ��������·�Ӧ��

����������Ϣ������������
��1��
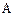 ��������__________��
������Ϊ��__________��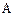 �ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________��
�ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________����2��д��
 ת��Ϊ
ת��Ϊ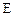 �Ļ�ѧ����ʽ____________________��
�Ļ�ѧ����ʽ____________________����3��
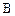 ��ͬ���칹��
��ͬ���칹��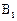 ������ֻ��һ���������ܷ���ˮ�ⷴӦ����
������ֻ��һ���������ܷ���ˮ�ⷴӦ����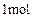
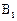 ������������Һ��Ӧ������ܵ�
������������Һ��Ӧ������ܵ�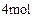
 ����
����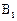 ���п��ܵĽṹ��ʽΪ��__________��
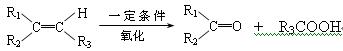
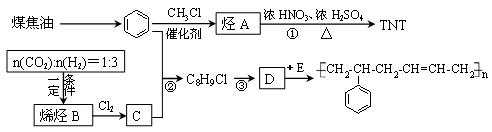
���п��ܵĽṹ��ʽΪ��__________����1��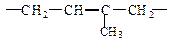 2������1��3������ϩ
2������1��3������ϩ
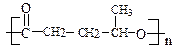 �ӳɷ�Ӧ��ԭ��Ӧ��ÿ��2�֣���8�֣�
�ӳɷ�Ӧ��ԭ��Ӧ��ÿ��2�֣���8�֣�
��2��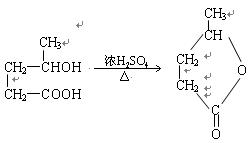 ��H2O ��2�֣�
��H2O ��2�֣�
��3��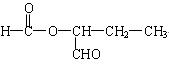 ��
��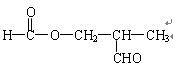 ��
��
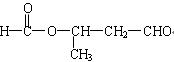 ��ÿ��2�֣���6�֣�
��ÿ��2�֣���6�֣�
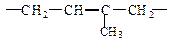 2������1��3������ϩ
2������1��3������ϩ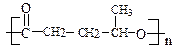 �ӳɷ�Ӧ��ԭ��Ӧ��ÿ��2�֣���8�֣�
�ӳɷ�Ӧ��ԭ��Ӧ��ÿ��2�֣���8�֣���2��
 ��H2O ��2�֣�
��H2O ��2�֣���3��
 ��
��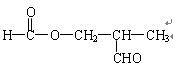 ��
��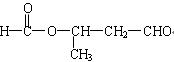 ��ÿ��2�֣���6�֣�
��ÿ��2�֣���6�֣���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

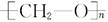 ����ͨ���Ӿ۷�Ӧ�Ƶõģ��䵥����HCHO
����ͨ���Ӿ۷�Ӧ�Ƶõģ��䵥����HCHO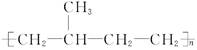 �ĵ�����
�ĵ�����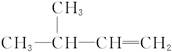
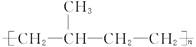 ����������ֱ�Ӵ����ͽṹ��Ϊ���ͽṹ
����������ֱ�Ӵ����ͽṹ��Ϊ���ͽṹ ��������������ֱ�������ͽṹ������ͽṹ
��������������ֱ�������ͽṹ������ͽṹ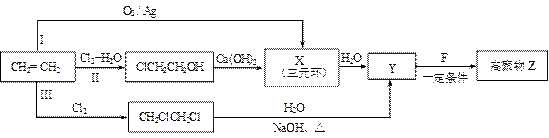
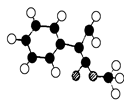
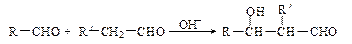 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��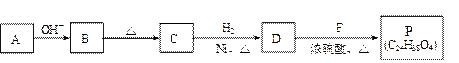
 ����ˮ��ΪM��N b��һ��������M����ת��ΪN
����ˮ��ΪM��N b��һ��������M����ת��ΪN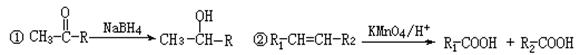
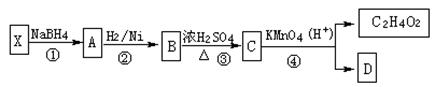
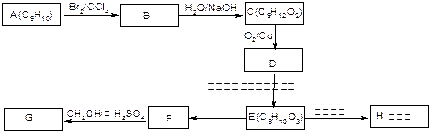
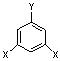 ��ʾ������X��Y����ΪH��
��ʾ������X��Y����ΪH��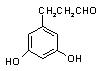 ��
��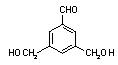 �� ��
�� ��  ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ���������
���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������� ���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��
���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��