��Ŀ����
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
��1���� �Ҷ��� �� �Լ�����
��2���� HOCH2CH2OH ��B�ķ���ʽΪ C3H6O B�Ľṹ��ʽΪ CH3CH2CHO
����:��

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ
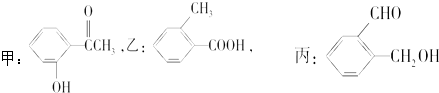
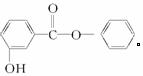
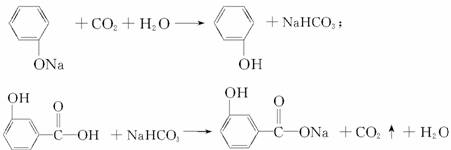
 ��CH3�� �� ��
��CH3�� �� �� ��CH3�� �� ��
��CH3�� �� ��