��Ŀ����
��15�֣�
ij��ѧ��ȤС���ͬѧΪ��ȡ���������飬�������ϵ�֪��
NaBr��H2SO4 HBr��NaHSO4
HBr��NaHSO4
CH3CH2OH��HBr CH3CH2Br��H2O
CH3CH2Br��H2O
��ѧ��ȤС�����ʵ��ԭ���������ͼ��װ�á�
�����������������գ��й������б����£�
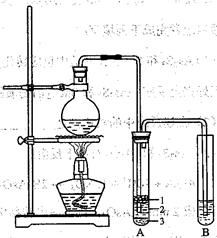
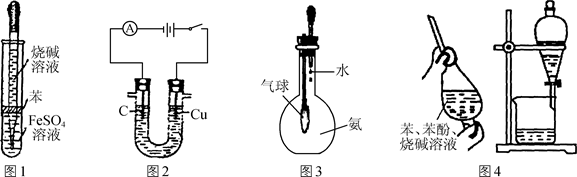
��1��Բ����ƿ�м���ķ�Ӧ�����廯�ơ� ��2:1�����ᡣ���������2��1���������õ�����Ϊ (ѡ���ţ�
a���ձ� b�������� c����Ͳ d������ƿ e.�ζ���
��2���������ﵼ��ʢ�б�ˮ�������Թ�A�У��Թ�A�е����ʷ�Ϊ���㣨��ͼ��ʾ���������ڵ�
�㣻
��3����Ũ���������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ��������Ӧ��
�� (ѡ���ţ�Ȼ���ٽ��� һ���������ɣ�
a����ˮ�Ȼ��� b����������Һ c�����Ȼ�̼ d������������Һ
��4��ʵ������У�ͬѧ�����������Ӳ�����©��������ʦ���������װ���е��������Ӳ��ֶ��ijɱ������ӿڣ���ԭ���ǣ� ��
��5���������ȡ�õ���ˮ�Ҵ���57.5mL,���õ��Ĵ�������������52.0mL����������IJ���Ϊ ��
ij��ѧ��ȤС���ͬѧΪ��ȡ���������飬�������ϵ�֪��
NaBr��H2SO4
 HBr��NaHSO4
HBr��NaHSO4 CH3CH2OH��HBr
 CH3CH2Br��H2O
CH3CH2Br��H2O��ѧ��ȤС�����ʵ��ԭ���������ͼ��װ�á�
�����������������գ��й������б����£�
| | �Ҵ� | ������ |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.8 | 1.4 |
| �е㣯�� | 78.5 | 119.0 |
| �۵㣯�� | -l30 | 38.4 |

��1��Բ����ƿ�м���ķ�Ӧ�����廯�ơ� ��2:1�����ᡣ���������2��1���������õ�����Ϊ (ѡ���ţ�
a���ձ� b�������� c����Ͳ d������ƿ e.�ζ���
��2���������ﵼ��ʢ�б�ˮ�������Թ�A�У��Թ�A�е����ʷ�Ϊ���㣨��ͼ��ʾ���������ڵ�
�㣻
��3����Ũ���������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ��������Ӧ��
�� (ѡ���ţ�Ȼ���ٽ��� һ���������ɣ�
a����ˮ�Ȼ��� b����������Һ c�����Ȼ�̼ d������������Һ
��4��ʵ������У�ͬѧ�����������Ӳ�����©��������ʦ���������װ���е��������Ӳ��ֶ��ijɱ������ӿڣ���ԭ���ǣ� ��
��5���������ȡ�õ���ˮ�Ҵ���57.5mL,���õ��Ĵ�������������52.0mL����������IJ���Ϊ ��
��1����ˮ�Ҵ���2�֣���abc��3�֣� ��2��3 ��2�֣�
��3��d��2�֣�����Һ ��2�֣� ��4����Ӧ�����Br2����ʴ��2�֣� ��5��66.8%��2�֣�
��3��d��2�֣�����Һ ��2�֣� ��4����Ӧ�����Br2����ʴ��2�֣� ��5��66.8%��2�֣�
�����������1��������Ŀ������Ϣ����Ӧ�ﻹ��Ҫ����ˮ�Ҵ�������Բ����ƿ�м���ķ�Ӧ�����NaBr�����ᣬ��Ҫ������ˮ�Ҵ������������2��1��������Ҫ��Ͳ��ȡŨ�����ˮ��ͬ�������Ҫ���ձ����ܽ⣬���ò��������裬�ʴ�Ϊabc��
��2����������ܶ��������ײ㣬����3�㡣
��3���Թ�A�л�õ��л�����ػ�ɫ��˵��������Br2��Br2���������ԣ���������������Һ��ȥ��Br2������������Һ��Ӧ�������������ˮ������ͨ����Һ������ɳ��ӡ�
��4��Ũ��������Br?����Br2��Br2���и�ʴ�ԣ��ܸ�ʴ�����������Ӳ��ֶ��ijɱ������ӿڡ�

��ϰ��ϵ�д�
�����Ŀ