��Ŀ����
����18�֣�I��6�֣�д�������Ȼ�ѧ��Ӧ����ʽ
��1��N2 (g)��H2(g)��Ӧ����1molNH3(g)���ų�46.1KJ������
��2��1molC2H5OH(l)��ȫȼ������CO2(g)��H2O(l)���ų�1366.8KJ������
��3��1molC��ʯī��������H2O(g)��Ӧ����131.3KJ����
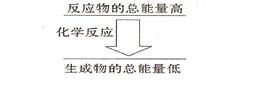
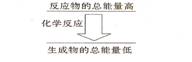
II.��12�֣���1����ѧ��Ӧ�о������������ı仯����ѧ���Ķ��Ѻ��γ��Ƿ��������仯����Ҫԭ���������л�ѧ���γ�ʱ��__________��������ų��������ա��������һ����ѧ��Ӧ����ѧ������ʱ�������仯���ڻ�ѧ���γ�ʱ�������仯����÷�Ӧ����_________��Ӧ�� ���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
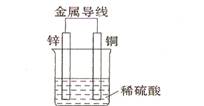
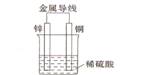
��2����ͬ��ʽ�����������ת�����磺��ѧ�ܺ͵��ܡ�����֮����ת������ͼ��һ��ԭ��ع���ԭ����ʾ��ͼ���Իش�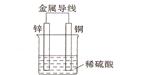
�ٴ������Ƕȿ���������____________��ת��Ϊ____________�ܣ�
��װ����ZnΪ____________����
��18�֣�I��1��1/2N2(g)��3/2H2(g)��NH3(g) ��H����46.1 kJ��mol-1
��2��C2H5OH(g)+3O2(g)==2CO2(g)+3H2O(l)����H����1366.8 kJ��mol-1
��3��C��ʯīs��+H2O(g)="=" CO(g)+ H2(g)����H��131.3 kJ��mol-1��ÿ��2�֣�
II��1���ų� ���� ���� ��2���ٻ�ѧ �� �ڸ� ��ÿ��2�֣�
����

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�