��Ŀ����
��������ʵ���г�����Ӧ������ȷ���� �� ��
ʵ �� �� ʵ | �� �� | |
�� | Cl2��ˮ��Һ���Ե��� | Cl2�ǵ���� |
�� | ��ȼ�յ�þ������CO2���ܼ���ȼ�� | ��ԭ�ԣ�Mg>C |
�� | NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ���� | ���ԣ�HCO3����Al��OH��3 |
�� | �����°�����ȼ���������ڷŵ�ʱ����������Ӧ | �ǽ����ԣ�P��N |
�� | ij��ɫ��Һ�м�������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ���� | ����Һһ����NH4�� |
A���ۢܢ� B���ڢۢ� C���٢ڢ� D��ȫ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��о�SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1�������Ƽ�ѭ�������ѳ������е�SO2�����Ƽ�ѭ�����У���Na2SO3��Һ��Ϊ����Һ����SO2�Ĺ����У�pH��n��SO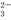 ����n��HSO
����n��HSO ���仯��ϵ���±���
���仯��ϵ���±���
n��SO | 91��9 | 1��1 | 9��91 |
pH | 8��2 | 7��2 | 6��2 |
�����ϱ��жϣ�NaHSO3��Һ��____________�ԣ���ƽ��ԭ�����ͣ�_____________________��
�ڵ�����Һ������ʱ����Һ������Ũ�ȹ�ϵ��ȷ���ǣ�ѡ����ĸ��___________________��
a��c��Na������2c��SO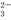 ����c��HSO
����c��HSO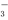 ��
��
b��c��Na����>c��HSO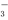 ��>c��SO
��>c��SO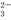 ��>c��H������c��OH����
��>c��H������c��OH����
c��c��Na������c��H������c��SO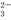 ����c��HSO
����c��HSO ����c��OH����
����c��OH����
��2����ij��Һ�к�3mol Na2SO3������һ������ϡ���ᣬǡ��ʹ��Һ��Cl����HSO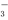 �����ʵ���֮��Ϊ2��1��������������HCl�����ʵ�������_____________mol��
�����ʵ���֮��Ϊ2��1��������������HCl�����ʵ�������_____________mol��
��3��CO�����ںϳ�CH3OH����Ӧ����ʽΪ��CO��g��+2H2��g�� CH3OH��g�� ,��һ���¶�ѹǿ�£����ݻ�Ϊ2L���ܱ�������ͨ��0��2molCO��0��4molH2����ƽ��ʱCO��ת����50%������¶��µ�ƽ�ⳣ��Ϊ ���ټ���1��0molCO�����´ﵽƽ�⣬��CO��ת���� ������������䡱��С������CH3OH��������� ������������䡱��С������
CH3OH��g�� ,��һ���¶�ѹǿ�£����ݻ�Ϊ2L���ܱ�������ͨ��0��2molCO��0��4molH2����ƽ��ʱCO��ת����50%������¶��µ�ƽ�ⳣ��Ϊ ���ټ���1��0molCO�����´ﵽƽ�⣬��CO��ת���� ������������䡱��С������CH3OH��������� ������������䡱��С������
��4����0��02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ������֪Ksp��BaSO4����1��1��10-10��
��5���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ��ʵ��������ͼװ��ģ���������̣���ԭ���ǣ�ͨ���Co2+������Co3+��Ȼ����Co3+����������ˮ�еļ״�������CO2��������Co3+�Ļ�ԭ������Co2+����
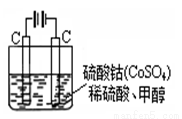
�� д�������缫��Ӧʽ�� ��
�� д����ȥˮ�еļ״������ӷ���ʽ�� ��
��Cl�������ʵ���Ũ��Ϊ0.5mol•L-1��ij��ɫ ������Һ�У������ܺ����������ʾ�����������ӣ�
������Һ�У������ܺ����������ʾ�����������ӣ�
������ | K+ Al3+ Mg2+ Ba2+ Fe3 |
������ | NO3- CO32- SiO32- SO42- OH- |
��ȡ����Һ200mL��������ʵ�飨����������ڱ�״���²ⶨ����
��� | ʵ������ | ʵ���� |
�� | �����Һ�м�������ϡ���� | ������ɫ�������ų���״����0.56L���� |
�� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4g |
�� | ������Һ�еμ�BaCl2��Һ | ���������� |
��ش��������⣮
��1��ͨ������ʵ�鲻��ȷ���Ƿ���ڵ�������
��2��ʵ�����������������ӷ���ʽΪ
��3��ͨ��ʵ���ͱ�Ҫ���㣬��д��һ�����ڵ��� ���Ӽ���Ũ�ȣ���һ��Ҫ��������
���Ӽ���Ũ�ȣ���һ��Ҫ��������
������ | Ũ��c/��mol/L�� |
��4���ж�K+�Ƿ���ڣ������ڣ��������СŨ�ȣ���������˵�����ɣ� _



 ʽΪC23H35O7��1mol����
ʽΪC23H35O7��1mol����
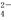 ��Cl����H2O��δ��ƽ���������й�˵������ȷ���ǣ� ��
��Cl����H2O��δ��ƽ���������й�˵������ȷ���ǣ� ��

 �ȼҵ����Ҫ��Ʒ��B�������ԣ���C��Һ�г�OH- ����ڵ������ӵĻ�ѧʽΪ________
�ȼҵ����Ҫ��Ʒ��B�������ԣ���C��Һ�г�OH- ����ڵ������ӵĻ�ѧʽΪ________