��Ŀ����
����Ŀ��ԭ���������������A��B��C��D��E��F����Ԫ�ء�����A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��Cλ��ͬһ���壬F��ԭ������Ϊ24��
(1)Fԭ�ӻ�̬�ļ۵����Ų�ʽΪ_______________��
(2)��A��B��C����Ԫ���У��縺���ɴ�С��˳����______>______>_____����Ԫ�ط��Żش𣩡�
(3)Ԫ��C�ļ���̬�⻯��ķе�Զ����Ԫ��E�ļ���̬�⻯��ķе㣬����Ҫԭ����________________________��
��4����A��B��C�γɵ�����CAB����AC2��Ϊ�ȵ����壬��CAB���Ŀռ乹��Ϊ_____________��
��5����Ԫ��A��E���γɵij����������У�Aԭ�ӹ�����ӻ�����Ϊ_______________��
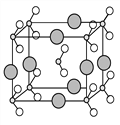
��6����B��C��D����Ԫ���γɵĻ����ᄃ��ľ�����ͼ��ʾ����û�����Ļ�ѧʽΪ________________________��
���𰸡� 1S22S22P63S23P63d54S1 O N C ˮ���Ӽ������� ֱ�� SP NaNO2
��������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ���A��C��C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ����C��O������B��N��DΪ�����ڵ�������ԭ�Ӱ뾶��������Ԫ�أ���D��Na��E��Cλ��ͬһ���壬E��S��F��ԭ������Ϊ24��F��Cr��(1)��ԭ�Ӻ�����Ӵ��ڸù����ȫ�������������ȫ��ʱ���ȶ���״̬������Fԭ�ӻ�̬�ĺ�������Ų�ʽΪ1S22S22P63S23P63d54S1��[Ar] 3d54S1��(2)һ������£�ͬһ���ڵ�Ԫ�أ�ԭ������Խ��Ԫ�صķǽ�����Խǿ��������ܾ�Խ���ǵ�ԭ�Ӻ�����Ӵ��������İ����״̬ʱ�����ȶ���״̬��ʧȥ���ӱ�ԭ��������1��Ԫ�صĻ���������A��B��C����Ԫ���У���һ�������ɴ�С��˳����N >O>C�� (3)Ԫ��B�ļ���̬�⻯��ķе�Զ����Ԫ��A�ļ���̬�⻯��ķе㣬NԪ�صķǽ�����ǿ��ԭ�Ӱ뾶С���ڷ��Ӽ�����ڷ��Ӽ��������⣬����������������˷��Ӽ������������4����A��B��C�γɵ�����CAB-����AC2��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ�����Ҳ���ƣ���CAB-�ĽṹʽΪ[N=C=O]-����5����Ԫ��A��E���γɵij���������CS2�У�Aԭ�ӹ�����ӻ�����Ϊsp�ӻ�����6����B��C��D����Ԫ���γɵĻ����ᄃ��ľ�������ͼ��ʾ������Na+��8��![]() =2������NO2-��8��
=2������NO2-��8��![]() +1=2�����Ըû�����Ļ�ѧʽΪNaNO2��
+1=2�����Ըû�����Ļ�ѧʽΪNaNO2��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
