��Ŀ����
 һ��������������ԭ��Ӧ����ʽ���Բ�д���������뷴Ӧʽ����һ���ǡ�������Ӧʽ����һ���ǡ���ԭ��Ӧʽ������2Fe3++Cu=2Fe2++Cu2+���ɲ�дΪ������Ӧʽ��Cu��Cu2++2e-����ԭ��Ӧʽ��2Fe3++2e-��2Fe2+�����ɴ�ʵ���˻�ѧ������ܵ��ת�����ݴˣ��ش��������⣺
һ��������������ԭ��Ӧ����ʽ���Բ�д���������뷴Ӧʽ����һ���ǡ�������Ӧʽ����һ���ǡ���ԭ��Ӧʽ������2Fe3++Cu=2Fe2++Cu2+���ɲ�дΪ������Ӧʽ��Cu��Cu2++2e-����ԭ��Ӧʽ��2Fe3++2e-��2Fe2+�����ɴ�ʵ���˻�ѧ������ܵ��ת�����ݴˣ��ش��������⣺��1������ӦZn+2H+�TZn2++H2����дΪ�������뷴Ӧʽ�������У�������ӦʽΪ��
Zn=Zn2++2e-
Zn=Zn2++2e-
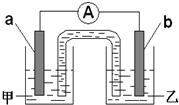
����2�����⣨1����Ӧ����Ƴ�ԭ�����ͼ��ʾ�����缫aΪZn���缫b��ѡ����ϣ�
ͭ
ͭ
��ֻ��һ�֣����������Һ����ZnSO4
ZnSO4
���缫b���ĵ缫��Ӧʽ��2H++2e-=H2��
2H++2e-=H2��
����3���ɷ�Ӧ2H2+O2
| ||
2H2-4e-=4H+
2H2-4e-=4H+
������O2+4H++4e-=2H2O
O2+4H++4e-=2H2O
����4���Զ��Բ���Ϊ�缫�����100mL pH=6������ͭ��Һ������������Һ��pHΪ1ʱ���缫��������ͭ������Ϊ
0.32g
0.32g
�����Ե��ǰ����Һ����ı仯������������1��ʧ���ӵ����ʷ���������Ӧ��
��2������ӦZn+2H+�TZn2++H2�������缫aΪZn���缫bΪ����п���õĽ�����ķǽ������缫����Ӧ�ĵ������Һ������ͬ�������ӣ������ϵõ��ӷ�����ԭ��Ӧ��
��3���ɷ�Ӧ2H2+O2
2H2O����Ƴ���ϡ����Ϊ�������Һ��ȼ�ϵ�أ�������ȼ��ʧ���ӷ���������Ӧ�������������õ��ӷ�����ԭ��Ӧ��
��4������ͭ��������ʵ���֮��Ĺ�ϵʽ���㣮
��2������ӦZn+2H+�TZn2++H2�������缫aΪZn���缫bΪ����п���õĽ�����ķǽ������缫����Ӧ�ĵ������Һ������ͬ�������ӣ������ϵõ��ӷ�����ԭ��Ӧ��
��3���ɷ�Ӧ2H2+O2
| ||
��4������ͭ��������ʵ���֮��Ĺ�ϵʽ���㣮
����⣺��1�����ݷ���ʽ֪��пʧ���ӷ���������Ӧ�������䷴ӦʽΪ��Zn=Zn2++2e-��
�ʴ�Ϊ��Zn=Zn2++2e-��
��2����ӦZn+2H+�TZn2++H2�������缫aΪZn���缫bΪ����п���õĽ�����ķǽ�������ͭ�ȣ��缫����Ӧ�ĵ������Һ������ͬ�������ӣ�����������п��Һ�������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��2H++2e-=H2����
�ʴ�Ϊ��Cu��ZnSO4��2H++2e-=H2����
��3���ɷ�Ӧ2H2+O2
2H2O����Ƴ���ϡ����Ϊ�������Һ��ȼ�ϵ�أ�������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2H2-4e-=4H+�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+4H++4e-=2H2O��
�ʴ�Ϊ��������2H2-4e-=4H+��������O2+4H++4e-=2H2O��
��4��������ͭ��������x��
�������ͭ��Һ�ķ���ʽΪ��2Cu 2++2H2O
2Cu��+O2��+4H+��
128g 4mol
x 0.1mol/L��0.1L
x=
=0.32g��
�𣺵缫��������ͭ������Ϊ0.32g��
�ʴ�Ϊ��Zn=Zn2++2e-��
��2����ӦZn+2H+�TZn2++H2�������缫aΪZn���缫bΪ����п���õĽ�����ķǽ�������ͭ�ȣ��缫����Ӧ�ĵ������Һ������ͬ�������ӣ�����������п��Һ�������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��2H++2e-=H2����
�ʴ�Ϊ��Cu��ZnSO4��2H++2e-=H2����
��3���ɷ�Ӧ2H2+O2
| ||
�ʴ�Ϊ��������2H2-4e-=4H+��������O2+4H++4e-=2H2O��
��4��������ͭ��������x��
�������ͭ��Һ�ķ���ʽΪ��2Cu 2++2H2O
| ||
128g 4mol
x 0.1mol/L��0.1L
x=
| 0.1mol/L��0.1L��128g |
| 4mol |
�𣺵缫��������ͭ������Ϊ0.32g��
���������⿼��ԭ���ԭ���͵���ԭ�����缫��Ӧʽ����д��ѧϰ�ѵ㣬��дʱҪ��ϵ������Һ������ԣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ