��Ŀ����
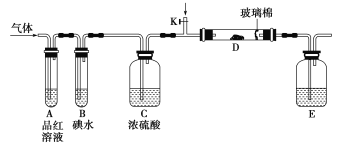
����Ŀ��Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ�������ͼ��ʾ��ʵ��װ�á�

(1)���巢��װ��B������������________��
(2)Ϊ��֤ͨ��װ��D�е�������Cl2��������SO2������ijС��ͬѧ���������Լ���
���Ȼ�������Һ�� �����軯����Һ�� ��Ʒ����Һ �����Ը��������Һ
a����Cl2������ȡ����D����Һ�μ���ʢ��__________(ѡ��һ�����)���Թ��ڣ��ټ���________(ѡ��һ�����)�Լ���ʵ��������_______________________________��
b����SO2������ȡ����D����Һ�μ���ʢ��__________(ѡ��һ�����)���Թ��ڣ�ʵ��������________________________________________��
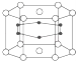
(3)��һС�������ͼ��ʾ��װ��ͼ(�гֺͼ���װ����ȥ)���ֱ��о�SO2��Cl2�����ʡ�
��������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ��_________(������ͬ����������ͬ��)��
����װ��B��װ��5.0 mL 1.0 mol��L��1��ˮ����ͨ������Cl2��ȫ��Ӧ��ת����0.05mol���ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________________��
(4)����Ԫ��S��O��ɣ�2���������X��X��S��O��������Ϊ4��3����Cl2�뺬X����Һ��ȫ��Ӧ����dz��ɫ�������ɣ�ȡ�ϲ���Һ�����Ȼ�����Һ���а�ɫ����������д��Cl2�뺬X����Һ��Ӧ�����ӷ���ʽ��_____________________________��
���𰸡�Cl2 �� �� ��Һ��Ѫ��ɫ �� ��Һ��ɫ������Һ��ɫ��dz�� ��ͬ 5Cl2��I2��6H2O=2HIO3��10HCl ![]() ��Cl2��H2O=S��+
��Cl2��H2O=S��+![]() ��2Cl����2H��
��2Cl����2H��
��������
��1�����巢��װ��B���������徭������ʳ��ˮ��Ž���װ�ã�˵��װ��AΪ��ȡ���������װ�ã�װ��BΪ��ȡ������װ�ã���Ϊ��Cl2��
��2������Cl2������ȡ����D����Һ�μ���ʢ���Ȼ�������Һ�Լ����Թ��ڣ��ټ������軯����Һ���Ѫ��ɫ��֤�����������������ӣ�
��Ϊ����������Һ��Ѫ��ɫ
����SO2������������SO2���л�ԭ�ԣ��������Ը��������Һ������Ӧʹ��ɫ���������Һ��ɫ���м��飻
��Ϊ��������Һ��ɫ������Һ��ɫ��dz��
��3���ٶ��������ܺ���ɫ���ʷ�Ӧ������ɫ���ʣ����Զ���������Ư���ԣ�������ˮ��Ӧ���ɴ����ᣬ���������ǿ�����ԣ���ʹ��ɫ������ɫ������������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽��������ͬ����ʹƷ����ɫ����Ϊ����ͬ��
����װ��B��װ��5.0mL1.0��10-3mol/L�ĵ�ˮ����ͨ������Cl2��ȫ��Ӧ��ת�Ƶĵ���Ϊ5.0��10-5mol�����Ԫ�������������еĻ��ϼ�Ϊa������ݵ���ת���غ���5.0��10-3L��1.0��10-3mol/L��2��a=5.0��10-5mol�����a=+5�����Եⵥ�ʱ�����ΪHIO3����÷�Ӧ�Ļ�ѧ����ʽΪ5Cl2+I2+6H2O=2HIO3+10HCl����Ϊ��5Cl2��I2��6H2O=2HIO3��10HCl��
��4������Ԫ��S��O���-2���������X��X��S��O��������Ϊ4:3������X��S��O��ԭ�Ӹ�����Ϊ432:316=3:2�����Ը�����ΪS2O32��������ǿ�����ԣ��ܺ������������ӷ���������ԭ��Ӧ����������֪���÷�Ӧ����������������ɣ�������������Ԫ�صõ������������ӣ�ͬʱ��dz��ɫ����������������S���ʣ����Ը÷�Ӧ�����ӷ���ʽΪ��Cl2+S2O32+H2O=SO42+S��+2Cl-+2H+��

 ����������ϵ�д�
����������ϵ�д�