��Ŀ����
����ʱ��2008��9��25��21ʱ10��04�룬�ҹ��������Ƶ������ߺ����˷ɴ��ھ�Ȫ���Ƿ������ķ������գ��������߷ɴ���������ij�������F���ػ��������ƽ�����ƫ������{����ʽ��C2H8N2}��������������
��1��6.0gҺ̬ƫ��������������Һ̬��������������ȫ��Ӧ����N2��g����CO2��g����H2O��g�����ų�225.0kJ��������
��д��������Ӧ���Ȼ�ѧ����ʽ�� ��
�������ж���ȷ����
A�����ʵ�ȼ�շ�Ӧ�����������μ�
B���÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ2��1
C��ƫ������ȼ����Ϊ2250kJ/mol
D��ƫ������������������������ƽ����Ƕ��߷������ҵ�������ԭ���ų��������ȺͲ������������ԭ��
��2��ʹ��ƫ��������������������ȼ�ϣ�ʹ���䳡�����ػ�ɫ��Ģ������������������Ա�����彡��������һЩ����֢�������ػ�ɫ��Ģ�����ƵĿ���ԭ�� ��
��3���µĻ�������ȼ�ϼƻ���2014�굽2016��ʹ�ã����ں��Ϸ��䳡�����е����������ʱ����ã��µĵ���ȼ����Һ�⣮���ú��ܰ�ˮ�ֽ⣬��ȡ��������Ŀǰ������������о��Ŀ��⣮��ͼ�ǹ��������о���һ�����̣���-����ѭ���������������˹����ĵ⣮
д���ٷ�Ӧ�Ļ�ѧ����ʽ ��
����-����ѭ������ȡ�������ŵ��� ��
��1��6.0gҺ̬ƫ��������������Һ̬��������������ȫ��Ӧ����N2��g����CO2��g����H2O��g�����ų�225.0kJ��������
��д��������Ӧ���Ȼ�ѧ����ʽ��
�������ж���ȷ����
A�����ʵ�ȼ�շ�Ӧ�����������μ�
B���÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ2��1
C��ƫ������ȼ����Ϊ2250kJ/mol
D��ƫ������������������������ƽ����Ƕ��߷������ҵ�������ԭ���ų��������ȺͲ������������ԭ��
��2��ʹ��ƫ��������������������ȼ�ϣ�ʹ���䳡�����ػ�ɫ��Ģ������������������Ա�����彡��������һЩ����֢�������ػ�ɫ��Ģ�����ƵĿ���ԭ��
��3���µĻ�������ȼ�ϼƻ���2014�굽2016��ʹ�ã����ں��Ϸ��䳡�����е����������ʱ����ã��µĵ���ȼ����Һ�⣮���ú��ܰ�ˮ�ֽ⣬��ȡ��������Ŀǰ������������о��Ŀ��⣮��ͼ�ǹ��������о���һ�����̣���-����ѭ���������������˹����ĵ⣮

д���ٷ�Ӧ�Ļ�ѧ����ʽ
����-����ѭ������ȡ�������ŵ���
��������1���ٸ������ʵ����ʵ��������������ȣ��Լ��Ȼ�ѧ����ʽ����д���������
��A�����ʵ�ȼ�շ�Ӧ��һ���������μӣ���Һ̬ƫ��������Һ̬������������ȼ�գ�
B���÷�Ӧ��������N2O4�뻹ԭ��C2H8N2���ʵ���֮��Ϊ2��1��
C��ȼ������ָ1mol��������ȫȼ�������ȶ���������ų���������
D��ƫ������������������������ƽ�������������ԭ���ų��������ȺͲ����������壻
��2�����ݷ�ӦN2O4��g��?2NO2��g���Լ���������Ի�ѧƽ���Ӱ�죻
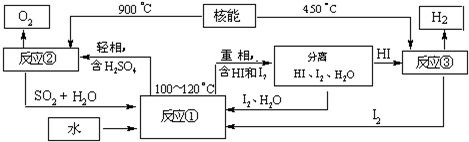
��3����ͼ֪��Ӧ��ΪSO2��I2��H2O��������ΪHI��H2SO4��Ȼ����������غ���ƽ�����ݸ÷�Ӧ��ѭ���Լ�����Ⱦ��
��A�����ʵ�ȼ�շ�Ӧ��һ���������μӣ���Һ̬ƫ��������Һ̬������������ȼ�գ�
B���÷�Ӧ��������N2O4�뻹ԭ��C2H8N2���ʵ���֮��Ϊ2��1��
C��ȼ������ָ1mol��������ȫȼ�������ȶ���������ų���������
D��ƫ������������������������ƽ�������������ԭ���ų��������ȺͲ����������壻
��2�����ݷ�ӦN2O4��g��?2NO2��g���Լ���������Ի�ѧƽ���Ӱ�죻
��3����ͼ֪��Ӧ��ΪSO2��I2��H2O��������ΪHI��H2SO4��Ȼ����������غ���ƽ�����ݸ÷�Ӧ��ѭ���Լ�����Ⱦ��
����⣺��1����6.0g��0.1molҺ̬ƫ��������������Һ̬��������������ȫ��Ӧ����N2��g����CO2��g����H2O��g�����ų�225.0kJ������������1molҺ̬ƫ��������������Һ̬��������������ȫ��Ӧ����N2��g����CO2��g����H2O��g�����ų�2250kJ�����������Ȼ�ѧ����ʽΪ��C2H8N2+2N2O4��l��=3N2��g��+2CO2��g��+4H2O��g����H=-2250kJ/mol���ʴ�Ϊ��C2H8N2��l��+2N2O4��l��=3N2��g��+2CO2��g��+4H2O��g����H=-2250kJ/mol��
��A�����ʵ�ȼ�շ�Ӧ��һ���������μӣ���Һ̬ƫ��������Һ̬������������ȼ�գ���A����
B���÷�Ӧ��������N2O4�뻹ԭ��C2H8N2���ʵ���֮��Ϊ2��1����B��ȷ��
C��N2���������������ȼ���ȵĸ���Ҫ��C����
D��ƫ������������������������ƽ����Ƕ��߷������ҵ�������ԭ���ų��������ȺͲ������������ԭ��D��ȷ��
��ѡ��BD��
��2�������Dz���Һ̬����������й¶�����������Ӧ����ȫ���£�����ƽ���ƶ�ԭ������ѹ�����´ٽ����淴Ӧƽ��N2O4��g��?2NO2��g��������ɫ������H��0�����ɶ������������ƶ���
�ʴ�Ϊ�������Dz���Һ̬����������й¶�����������Ӧ����ȫ���£�����ƽ���ƶ�ԭ������ѹ�����´ٽ����淴Ӧƽ��N2O4��g��?2NO2��g��������ɫ������H��0�����ɶ������������ƶ���
��3����ͼ֪��Ӧ��ΪSO2��I2��H2O��������ΪHI��H2SO4���ʷ���ʽΪ��SO2+I2+2H2O
2HI+H2SO4���÷�Ӧ��ѭ���ԣ�������ú��ܣ�������Ⱦ��
�ʴ�Ϊ��SO2+I2+2H2O
2HI+H2SO4��SO2��I2��ѭ��ʹ�ã�������ú��ܣ�����Ⱦ��
��A�����ʵ�ȼ�շ�Ӧ��һ���������μӣ���Һ̬ƫ��������Һ̬������������ȼ�գ���A����
B���÷�Ӧ��������N2O4�뻹ԭ��C2H8N2���ʵ���֮��Ϊ2��1����B��ȷ��
C��N2���������������ȼ���ȵĸ���Ҫ��C����
D��ƫ������������������������ƽ����Ƕ��߷������ҵ�������ԭ���ų��������ȺͲ������������ԭ��D��ȷ��
��ѡ��BD��
��2�������Dz���Һ̬����������й¶�����������Ӧ����ȫ���£�����ƽ���ƶ�ԭ������ѹ�����´ٽ����淴Ӧƽ��N2O4��g��?2NO2��g��������ɫ������H��0�����ɶ������������ƶ���
�ʴ�Ϊ�������Dz���Һ̬����������й¶�����������Ӧ����ȫ���£�����ƽ���ƶ�ԭ������ѹ�����´ٽ����淴Ӧƽ��N2O4��g��?2NO2��g��������ɫ������H��0�����ɶ������������ƶ���
��3����ͼ֪��Ӧ��ΪSO2��I2��H2O��������ΪHI��H2SO4���ʷ���ʽΪ��SO2+I2+2H2O
| ||
�ʴ�Ϊ��SO2+I2+2H2O
| ||
���������⿼���Ȼ�ѧ����ʽ����д����ѧƽ����ƶ�ԭ��������Դ��ʹ�ã��ѶȲ���

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
|
����ʱ��2008��9��25����9ʱ10�֣��й��������Ƶĵ��������˷ɴ������ߺţ��ھ�Ȫ���Ƿ����������˺��췢�䳡�ɡ���������F�����ػ���������գ��ӷ����ֳ����Կ������ӻ�����²�����������ĺ���ɫ�����壬�������ֺ���ɫ����ʶ��ȷ���ǣ� | |
A�� |
����������������NaOH��Һ���գ� |
B�� |
��NO2����������NaOH��Һ���գ� |
C�� |
����ۣ���������CS2��Һ���գ� |
D�� |
�ǹ������Ʒ�ĩ����������ϡ������Һ���գ� |