��Ŀ����
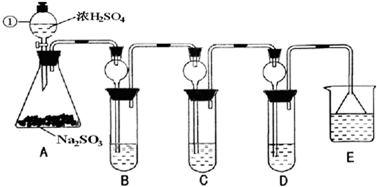
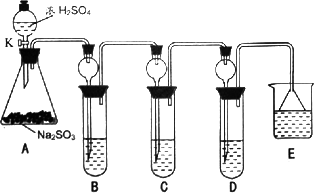
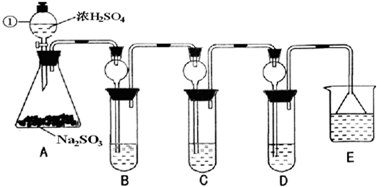
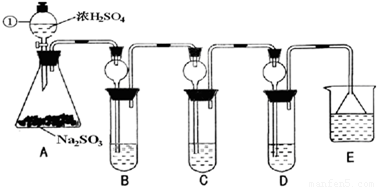
ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
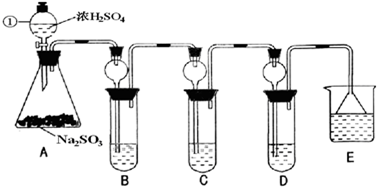
��1��ָ�������ٵ�����______��
��2�����Aװ�õ������Եķ�����______��
��3��װ��B����SO2�������ԣ���B����ʢ�Լ�����Ϊ______��
��4��װ��C��ʢװ��ˮ���Լ���SO2��______�ԣ���C�з�Ӧ�����ӷ���ʽΪ______��
��5��װ��D��ʢװ����Ư��Ũ��Һͨ��SO2һ�΅����D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
�ټ���һ���ð�ɫ����ΪCaSO3
��������ð�ɫ����Ϊ______��
���������ð�ɫ����Ϊ�����������ʵĻ���
�ڻ��ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5mol��L-1��HCl��0.5mol��L-1 H2SO4��0.5mol��L-1BaCl2��1mol��L-1 NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������ã�
��ش�ϴ�ӳ����ķ�����______��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������______���Լ��������ϴ����ܵĵ������������ܵ���һ�˲���ʢ��______���Լ������Թ��У�
������______���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��______��
��6��װ��E��ʢ�ŵ��Լ���______��������______��
�⣺��1���������dz��õ�������Һ©��������װ��������Һ��ҩƷ�����ã����ܿ���Һ���������Ӷ����Ʒ�Ӧ�����ʣ�
�ʴ�Ϊ����Һ©����
��2��װ�������Լ����ԭ���ǣ�ͨ�����巢�����븽���Һ�幹�ɷ����ϵ�����ݸı���ϵ��ѹǿʱ���������������ݵ����ɡ�ˮ�����γɡ�Һ��������ȣ����ж�װ�������Եĺû������Լ��װ��A�������ԵIJ���Ϊ�رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A���������ã�
�ʴ�Ϊ���رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A���������ã�
��3������ˮ��Һ�����ơ����⻯����Һ�е���Ԫ�ض�Ϊ-2�ۣ����������Ӧ�����ϼۻ����ߣ�����������������������ԣ��磺2H2S+SO2=3S+2H2O�У�H2S��SԪ�صĻ��ϼ����ߣ���������SO2��SԪ�صĻ��ϼ۽��ͣ�����ԭ��SO2Ϊ��������
�ʴ�Ϊ������ˮ��Һ�������ơ����⻯����Һ����
��4���ڷ�ӦBr2+SO2+2H2O�T2HBr+H2SO4�У�BrԪ�صĻ��ϼ���0����Ϊ-1�ۣ���Br2Ϊ���������ڷ�Ӧ�б��������ԣ�SԪ�صĻ��ϼ�+4������+6�ۣ���SO2Ϊ��ԭ�����ڷ�Ӧ�б������������ӷ�Ӧ����ʽ�У����ʺ�������д��ѧʽ���������ӷ���ʽΪ��SO2+Br2+2H2O=SO42-+4H++2Br-��
�ʴ�Ϊ����ԭ��SO2+Br2+2H2O=SO42-+4H++2Br-��
��5��������Ư��Ũ��Һ�к��еĴ���������Ӿ���ǿ�����ԣ�����������л�ԭ�ԣ��ᷢ��������ԭ��Ӧ�����ɲ���Ϊ����ƣ�
�ʴ�Ϊ��CaSO4��
�ڰ�ɫ���������и����Ӻ������ӡ���������ӵȿ����Ե����ӣ����ȥ��Щ���ӣ��������ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ�����������ƺ����ᷴӦCaSO3+2HCl�TCaCl2+SO2��+H2O������������Ʒ�컯��������ɫ���ʣ���ʹƷ����Һ��ɫ��Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�������+4�۵����������ɸð�ɫ�����Ļ�ѧ����ʽ��Ca��ClO��2+H2O+SO2=CaSO4+2HCl��
�ʴ�Ϊ���ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ�������������������0.5 mol?L-1HCl�� Ʒ����Һ�� ��������ȫ�ܽ⣬�����ݲ���������ʹƷ����Һ��ɫ��Ca��ClO��2+H2O+SO2=CaSO4+2HCl��
��6�������������ж������壬�Ǵ�����Ⱦ�����װ��E�����������ն��������ֹ��ɿ�����Ⱦ�������������ƣ��������ƺͶ�������Ӧ2NaOH+SO2�TNa2SO3+H2O��Ϊ��ֹ�����õ��۵�©����
�ʴ�Ϊ��NaOH��Һ������SO2����ֹ��ɿ�����Ⱦ��
��������1������ʵ���ҳ������������ƽ����ʵ���е����ý��н��
��2������װ�������Լ����ԭ�����
��3������������ԭ��Ӧ�Ĺ��ɣ�Ԫ�ػ��ϼ۽��ͷ�����ԭ��Ӧ�����������ԣ��ݴ˽��
��4��������ԭ��ӦBr2+SO2+2H2O�T2HBr+H2SO4�У�Br2�ڷ�Ӧ���������������������ԣ�����ԭ���������ӷ���ʽ����д������д���ӷ�Ӧ����ʽ���ݴ˽��
��5��������Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�����������������ӣ�
��������ı����п����Ե����ʣ���������ˮ�ظ�ϴ�ӣ�������ƺ����ᷴӦ���ɶ����������������Ư���ԣ���ʹƷ����ɫ��Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�������+4�۵��ݴ���д��ѧ����ʽ��
��6������NaOH��Һ����SO2�ͷ�������ԭ�����н��
������������Ҫ������̽��SO2�Ļ�ѧ����ʵ�����������ʵ���ԭ������������������ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�
�ʴ�Ϊ����Һ©����
��2��װ�������Լ����ԭ���ǣ�ͨ�����巢�����븽���Һ�幹�ɷ����ϵ�����ݸı���ϵ��ѹǿʱ���������������ݵ����ɡ�ˮ�����γɡ�Һ��������ȣ����ж�װ�������Եĺû������Լ��װ��A�������ԵIJ���Ϊ�رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A���������ã�
�ʴ�Ϊ���رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A���������ã�
��3������ˮ��Һ�����ơ����⻯����Һ�е���Ԫ�ض�Ϊ-2�ۣ����������Ӧ�����ϼۻ����ߣ�����������������������ԣ��磺2H2S+SO2=3S+2H2O�У�H2S��SԪ�صĻ��ϼ����ߣ���������SO2��SԪ�صĻ��ϼ۽��ͣ�����ԭ��SO2Ϊ��������
�ʴ�Ϊ������ˮ��Һ�������ơ����⻯����Һ����
��4���ڷ�ӦBr2+SO2+2H2O�T2HBr+H2SO4�У�BrԪ�صĻ��ϼ���0����Ϊ-1�ۣ���Br2Ϊ���������ڷ�Ӧ�б��������ԣ�SԪ�صĻ��ϼ�+4������+6�ۣ���SO2Ϊ��ԭ�����ڷ�Ӧ�б������������ӷ�Ӧ����ʽ�У����ʺ�������д��ѧʽ���������ӷ���ʽΪ��SO2+Br2+2H2O=SO42-+4H++2Br-��
�ʴ�Ϊ����ԭ��SO2+Br2+2H2O=SO42-+4H++2Br-��
��5��������Ư��Ũ��Һ�к��еĴ���������Ӿ���ǿ�����ԣ�����������л�ԭ�ԣ��ᷢ��������ԭ��Ӧ�����ɲ���Ϊ����ƣ�
�ʴ�Ϊ��CaSO4��
�ڰ�ɫ���������и����Ӻ������ӡ���������ӵȿ����Ե����ӣ����ȥ��Щ���ӣ��������ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ�����������ƺ����ᷴӦCaSO3+2HCl�TCaCl2+SO2��+H2O������������Ʒ�컯��������ɫ���ʣ���ʹƷ����Һ��ɫ��Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�������+4�۵����������ɸð�ɫ�����Ļ�ѧ����ʽ��Ca��ClO��2+H2O+SO2=CaSO4+2HCl��
�ʴ�Ϊ���ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ�������������������0.5 mol?L-1HCl�� Ʒ����Һ�� ��������ȫ�ܽ⣬�����ݲ���������ʹƷ����Һ��ɫ��Ca��ClO��2+H2O+SO2=CaSO4+2HCl��
��6�������������ж������壬�Ǵ�����Ⱦ�����װ��E�����������ն��������ֹ��ɿ�����Ⱦ�������������ƣ��������ƺͶ�������Ӧ2NaOH+SO2�TNa2SO3+H2O��Ϊ��ֹ�����õ��۵�©����
�ʴ�Ϊ��NaOH��Һ������SO2����ֹ��ɿ�����Ⱦ��
��������1������ʵ���ҳ������������ƽ����ʵ���е����ý��н��
��2������װ�������Լ����ԭ�����
��3������������ԭ��Ӧ�Ĺ��ɣ�Ԫ�ػ��ϼ۽��ͷ�����ԭ��Ӧ�����������ԣ��ݴ˽��
��4��������ԭ��ӦBr2+SO2+2H2O�T2HBr+H2SO4�У�Br2�ڷ�Ӧ���������������������ԣ�����ԭ���������ӷ���ʽ����д������д���ӷ�Ӧ����ʽ���ݴ˽��
��5��������Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�����������������ӣ�
��������ı����п����Ե����ʣ���������ˮ�ظ�ϴ�ӣ�������ƺ����ᷴӦ���ɶ����������������Ư���ԣ���ʹƷ����ɫ��Ư��Ũ��Һ�к��еĴ���������Ӿ��������ԣ�������+4�۵��ݴ���д��ѧ����ʽ��
��6������NaOH��Һ����SO2�ͷ�������ԭ�����н��
������������Ҫ������̽��SO2�Ļ�ѧ����ʵ�����������ʵ���ԭ������������������ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
�����Ŀ