��Ŀ����
25 ��ʱ����ˮ��D2O�������ӻ�Ϊ1.6��10-15��Ҳ����pHһ���Ķ������涨�����ȣ�pD=-lgc(D+)�������й�pD��������ȷ����
������D2O��pDΪ7.0 ����1 L D2O�У��ܽ�0.01 mol NaOD������pDΪ12 ��1 L 0.01 mol��L-1��DCl����ˮ��Һ�У�pD="2 " ����100 mL 0.25 mol��L-1 DCl����ˮ��Һ�У�����50 mL 0.2 mol��L-1 NaOD����ˮ��Һ����Ӧ����Һ��pD="1"
- A.�٢�
- B.�ۢ�
- C.�٢�
- D.�ڢ�
B
������ʱ��Ҫ���ڰ�ˮ�����ӻ�����ҺpH֪ʶǨ�ƹ������������龰���⡣����ˮ�д���ƽ�⣺D2O D++OD-������ˮʱc(D+)=c(OD-)=
D++OD-������ˮʱc(D+)=c(OD-)= mol��L-1=4��10-8 mol��L-1��
mol��L-1=4��10-8 mol��L-1��
pD=-lg(4��10-8)=7.40��7,�ʢٴ���
��c(OD-)="0.01" mol/1 L="0.01" mol��L-1,c(D+)=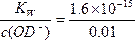 mol��L-1=1.6��10-13 mol��L-1,
mol��L-1=1.6��10-13 mol��L-1,
pD=-lg1.6��10-13=13-lg1.6��12
��c(D+)="0.01" mol��L-1,pD=2
��c(D+)=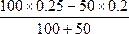 mol��L-1="0.1" mol��L-1,����pD=1��
mol��L-1="0.1" mol��L-1,����pD=1��
������ʱ��Ҫ���ڰ�ˮ�����ӻ�����ҺpH֪ʶǨ�ƹ������������龰���⡣����ˮ�д���ƽ�⣺D2O
 D++OD-������ˮʱc(D+)=c(OD-)=
D++OD-������ˮʱc(D+)=c(OD-)= mol��L-1=4��10-8 mol��L-1��
mol��L-1=4��10-8 mol��L-1��pD=-lg(4��10-8)=7.40��7,�ʢٴ���
��c(OD-)="0.01" mol/1 L="0.01" mol��L-1,c(D+)=
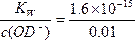 mol��L-1=1.6��10-13 mol��L-1,
mol��L-1=1.6��10-13 mol��L-1,pD=-lg1.6��10-13=13-lg1.6��12
��c(D+)="0.01" mol��L-1,pD=2
��c(D+)=
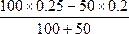 mol��L-1="0.1" mol��L-1,����pD=1��
mol��L-1="0.1" mol��L-1,����pD=1�� 
��ϰ��ϵ�д�
�����Ŀ