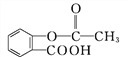
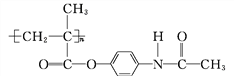
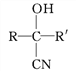
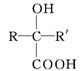
��Ŀ����
����Ŀ����Ҫ�������
��1����������������ͬλ�ص���__________������ͬ�����������__________��
A��O2��O3 B�� ![]() Cl��
Cl��![]() Cl
Cl
C��Fe2+��Fe3+ D��H2O��D2O��T2O
��2���ݱ�����ijЩ�����һ���������Ե�ϡ������뱣��Ӷ�����������˺����뽫����Rn��ԭ�ӽṹʾ��ͼ��ȫ��_______
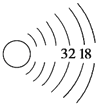
��3������������������Ar����CO2����K2S����NaOH��ֻ�д��ڹ��ۼ���������___�������ţ��õ���ʽ��ʾ�۵��γɹ�����__________��
��4�������ε���ɱȽϸ��ӣ��������������ʽ��ʾ��ʯ��Mg3Ca(SiO3)4��ʾΪ��������ʽΪ��__________��
��5����K37ClO3+6H35C1===KCl+3Cl2��+3H2O����˷�Ӧ��������������Է�������ԼΪ_________��������������λ��Ч���֣���
��6����NaHSO4����ˮ���ƻ���NaHSO4�е�______���ѧ�����ͣ���NaHSO4������״̬�µ��룬�ƻ���______���ѧ�����ͣ���д����������״̬�µĵ��뷽��ʽ_____________��
���𰸡� B A  ��
�� ![]() CaO��3MgO��4SiO2 70.7 ���ۼ� ���Ӽ� ���Ӽ� NaHSO4==Na++HSO4-
CaO��3MgO��4SiO2 70.7 ���ۼ� ���Ӽ� ���Ӽ� NaHSO4==Na++HSO4-
����������1��A��O2��O3��OԪ�صIJ�ͬ���ʣ�����ͬ��������B�� ![]() Cl��
Cl��![]() Cl����������ͬ����������ͬ������ͬλ�أ�C��Fe2+��Fe3+����ͬ��Ԫ�صIJ�ͬ���ӣ�D��H2O��D2O��T2O����ˮ�IJ�ͬ���ӣ��ʴ�Ϊ��B��A��
Cl����������ͬ����������ͬ������ͬλ�أ�C��Fe2+��Fe3+����ͬ��Ԫ�صIJ�ͬ���ӣ�D��H2O��D2O��T2O����ˮ�IJ�ͬ���ӣ��ʴ�Ϊ��B��A��
��2��Rn��86��Ԫ�أ����ݺ�����ӵ��Ų����ɣ�Rn��ԭ�ӽṹʾ��ͼΪ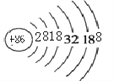 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3����Ar����ϡ�����壬�����ڻ�ѧ������CO2���ڹ��ۻ����ֻ���ڼ��Թ��ۼ�����K2S�������ӻ����ֻ�������Ӽ�����NaOH�������ӻ�����������Ӽ���H-O����ڼ��Թ��ۼ������ֻ�д��ڹ��ۼ��������Ǣ����õ���ʽ��ʾK2S���γɹ��̱�ʾΪ![]() ���ʴ�Ϊ������
���ʴ�Ϊ������![]() ��
��
��4��ʯ��Mg3Ca(SiO3)4����������ʽ��ʾΪCaO��3MgO��4SiO2���ʴ�Ϊ��CaO��3MgO��4SiO2��
��5����K37ClO3+6H35C1===KCl+3Cl2��+3H2O����˷�Ӧ�����ɵ������к���![]() ��
��![]() ��
��![]() ���֣����ݵ�ʧ�����غ㣬
���֣����ݵ�ʧ�����غ㣬 ![]() ��
��![]() �ĸ�����Ϊ1:5�������ɵ���������Է�������ԼΪ2����
�ĸ�����Ϊ1:5�������ɵ���������Է�������ԼΪ2����![]() ��37+
��37+![]() ��35��=70.7���ʴ�Ϊ��70.7��
��35��=70.7���ʴ�Ϊ��70.7��
��6����NaHSO4����ˮ���������Ƶ���������ӡ������Ӻ���������ӣ��ƻ���NaHSO4�е����Ӽ����ۼ���NaHSO4������״̬�µ��룬����������Ӻ�����������ӣ��ƻ������Ӽ���������״̬�µĵ��뷽��ʽΪNaHSO4=Na++HSO4-���ʴ�Ϊ�����ۼ� ���Ӽ������Ӽ���NaHSO4=Na++HSO4-��

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�����Ŀ��SO2���Ǵ�����Ⱦ��֮һ����Ҳ����Ҫ�Ĺ�ҵԭ�ϣ�ijͬѧ��ʵ�����������ʵ�飬��SO2�IJ������ʽ�����̽����
��1�����������ˮ��Һ
��SO2������ˮ�����³�ѹ���ܽ��Ϊ1:40��������H2SO3���ɡ���SO2�ı�����Һ�м���NaHSO3���壬������ð����ԭ����__________��������й�ƽ�ⷽ��ʽ��Ҫ˵����
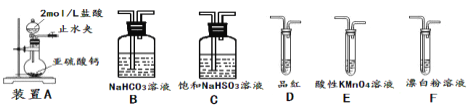
������֤���ԣ�H2SO3>HClO��ѡ�������װ�ã�������˳��Ϊ��
A![]() ________�������������ô�д��ĸ��ʾ���ɣ�����֤��H2SO3������ǿ��HClO��ʵ������Ϊ___��
________�������������ô�д��ĸ��ʾ���ɣ�����֤��H2SO3������ǿ��HClO��ʵ������Ϊ___��
��2����������Ļ�ԭ��
��֪SO2���л�ԭ�ԣ����Ի�ԭI2��������Na2O2������Ӧ����ͼʾװ�ý���ʵ�顣�����̶ֹ�װ��δ������
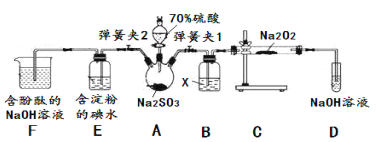
�������� | ʵ������ | ����ԭ�� |
�رյ��ɼ�2�����ɼ�1��ע����������û������ƿ�й��� | ���������ǵ�ľ������D�Թܿڴ���ľ������ȼ | SO2��Na2O2��Ӧ��O2���ɣ����ܷ����Ļ�ѧ��Ӧ����ʽΪ��____ |
���������ǵ�ľ������D�Թܿڴ���ľ����ȼ | SO2��Na2O2��Ӧ��O2���ɣ������Ļ�ѧ��ӦΪ��2SO2+2Na2O2=2Na2SO3+O2 | |
�رյ��ɼ�1�����ɼ�2�������������E��F�С� | E�Т�__________ | E�з�Ӧ�����ӷ���ʽ��________ |
F�Т�__________ | F�з�ӦΪ2OH-+SO2=SO32-+H2O |