��Ŀ����
 ������ʴ��ɺܴ�ľ�����ʧ������������Ȼ�ֺ������и�����ʴ��Ϊ���أ�
������ʴ��ɺܴ�ľ�����ʧ������������Ȼ�ֺ������и�����ʴ��Ϊ���أ���1��������������Һ�и�ʴʱ�������ĵ缫��ӦʽΪ
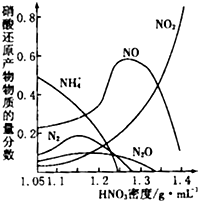
��2�������Ķۻ������Ǵﵽ��������Ŀ�ķ���֮һ�������ŨHNO3�������������һ����������Ĥ�����ڼ��Ȼ�ϡHNO3�оͲ��ܡ��ۻ�������ͬŨ�ȵ�HNO3������Ӧ��ԭ���︴�ӣ���ֲ�������ͼ��ʾ��
����ͼʾ��֪���ܶ�Ϊ1.05g?L-1��HNO3������Ӧ��ԭ������Ҫ��
����ͼʾ������֪��HNO3������Ӧʱ���������ܶȵ�����������Ҫ��ԭ����Ĺ�����
����mgFe�뺬ngHNO3��������Һǡ����ȫ��Ӧ��HNO3�Ļ�ԭ����ֻ��NO����m��ȡֵ��Χ��
��3��������M���ĸ�ʴ����Ϊ������Ӧ��M-ne-��Mn+�������������洦���ķ����⣬���з���Ҳ���������õ���
A�������в���������Ƚ������ı����ڲ��ṹ���Ƴɲ����
B��������Ʒ�����п
C����ˮ����բ����ֱ����Դ�ĸ�������
D��������к�̼�����Ƴ�������
��������1�����������Ի����·���������ʴ��
��2���ٸ���ͼ��֪���������£�������Ҫ����ԭΪNH4+����Ũ���£�������Fe�����ᷴӦ������������������李�ˮ��
�ڸ��ݻ�ԭ�����е�Ԫ�ػ��ϼ۷������
������������ʱ���ܽ�Fe��������С��������������ʱ�ܽ�Fe����������ݵ���ת���غ��ʾ��NO�����ʵ������ٸ���Nԭ���غ�ȷ��m��n��ϵ���ݴ˽��
��3����������ʴ�ķ����У��ı��ڲ��ṹ�ƳɺϽ���֣����DZ����㣬������������������������ӵ����������������ȣ�
��2���ٸ���ͼ��֪���������£�������Ҫ����ԭΪNH4+����Ũ���£�������Fe�����ᷴӦ������������������李�ˮ��
�ڸ��ݻ�ԭ�����е�Ԫ�ػ��ϼ۷������
������������ʱ���ܽ�Fe��������С��������������ʱ�ܽ�Fe����������ݵ���ת���غ��ʾ��NO�����ʵ������ٸ���Nԭ���غ�ȷ��m��n��ϵ���ݴ˽��
��3����������ʴ�ķ����У��ı��ڲ��ṹ�ƳɺϽ���֣����DZ����㣬������������������������ӵ����������������ȣ�
����⣺��1�����������Ի����·���������ʴ��������ӦʽΪO2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��2���ٸ���ͼ��֪���������£�������Ҫ����ԭΪNH4+����Ũ���£�������Fe�����ᷴӦ������������������李�ˮ����Ӧ���ӷ���ʽΪ��4Fe+10H++NO3-=4Fe2++NH4++3H2O���ʴ�Ϊ��NH4+��4Fe+10H++NO3-=4Fe2++NH4++3H2O��
����ͼ��֪�������Ũ��Խ���仹ԭ�����е�Ԫ�ػ��ϼ�Խ�ߣ��ʴ�Ϊ�������Ũ��Խ���仹ԭ�����е�Ԫ�ػ��ϼ�Խ�ߣ�
������������ʱ���ܽ�Fe��������С�����ݵ���ת���غ�n��NO��=
=
mol�����ݵ�ԭ���غ��֪n��HNO3��=n��NO��+3n[Fe��NO3��3]����
mol+3��
mol=
��������m=
n��
������������ʱ�ܽ�Fe����������ݵ���ת���غ�n�䣨NO��=
=
mol�����ݵ�ԭ���غ��֪n��HNO3��=n��NO��+2n[Fe��NO3��2]����
mol+2��
mol=
��������m=
n��
��m��ȡֵ��ΧΪ��
n��m��
n��
�ʴ�Ϊ��
n��m��
n��
��3����������ʴ�ķ����У��ı��ڲ��ṹ�ƳɺϽ���֣����DZ����㣬������������������������ӵ����������������ȣ���ABC��ȷ��D����ѡABC��
��2���ٸ���ͼ��֪���������£�������Ҫ����ԭΪNH4+����Ũ���£�������Fe�����ᷴӦ������������������李�ˮ����Ӧ���ӷ���ʽΪ��4Fe+10H++NO3-=4Fe2++NH4++3H2O���ʴ�Ϊ��NH4+��4Fe+10H++NO3-=4Fe2++NH4++3H2O��
����ͼ��֪�������Ũ��Խ���仹ԭ�����е�Ԫ�ػ��ϼ�Խ�ߣ��ʴ�Ϊ�������Ũ��Խ���仹ԭ�����е�Ԫ�ػ��ϼ�Խ�ߣ�
������������ʱ���ܽ�Fe��������С�����ݵ���ת���غ�n��NO��=
| ||
| (5-2) |
| m |
| 56 |
| m |
| 56 |
| m |
| 56 |
| ng |
| 63g/mol |
| 2 |
| 9 |
������������ʱ�ܽ�Fe����������ݵ���ת���غ�n�䣨NO��=
| ||
| 5-2 |
| m |
| 84 |
| m |
| 84 |
| m |
| 56 |
| ng |
| 63g/mol |
| 1 |
| 3 |
��m��ȡֵ��ΧΪ��
| 2 |
| 9 |
| 1 |
| 3 |
�ʴ�Ϊ��
| 2 |
| 9 |
| 1 |
| 3 |
��3����������ʴ�ķ����У��ı��ڲ��ṹ�ƳɺϽ���֣����DZ����㣬������������������������ӵ����������������ȣ���ABC��ȷ��D����ѡABC��
���������⿼�������ɸ�ʴ�������������ѧ��Ӧͼ���û�ѧ�����ѧ����ȣ��Ѷ��еȣ���2���м���ע�����ü���ȷ��m��ȡֵ��Χ��

��ϰ��ϵ�д�
�����Ŀ
 ������ʴ��ɺܴ�ľ�����ʧ������������Ȼ�ֺ������и�����ʴ��Ϊ���أ�Ѱ���ֹ������ʴ�ķ��������ش�
������ʴ��ɺܴ�ľ�����ʧ������������Ȼ�ֺ������и�����ʴ��Ϊ���أ�Ѱ���ֹ������ʴ�ķ��������ش�
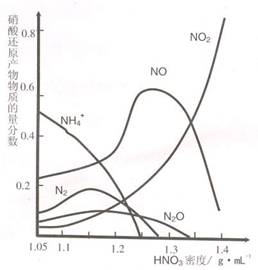 ��2�������ġ��ۻ�������Ҳ�ܴﵽ����Ŀ�ģ���
��2�������ġ��ۻ�������Ҳ�ܴﵽ����Ŀ�ģ���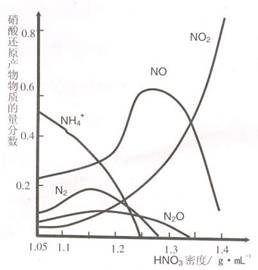 ��2�������ġ��ۻ�������Ҳ�ܴﵽ����Ŀ�ģ���
��2�������ġ��ۻ�������Ҳ�ܴﵽ����Ŀ�ģ���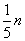 B��
B��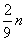 C��
C��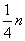 D��
D��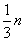 E��
E��