��Ŀ����
���õ�����CuSO4?5H2O������480mL 0.1mol?L-1��CuSO4��Һ������������գ�
��1����ͼ��ʾ����������������Һ�϶������õ�����______������ĸ��������������Һ����Ҫ�IJ���������______�����������ƣ���
��2�����Ƹ���ҺӦѡ��______ mL����ƿ��ʹ������ƿ֮ǰ�������______��
��3�����Ƹ���ҺӦ��������ƽ��ȡ______ g������
��4��ʹ������ƿ������Һʱ�����ڲ��������������������������ʹ������ҺŨ��ƫ�͵���______�����ţ���
������ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ���
��������ƿ��ת����Һʱ������Һ����������ƿ����
����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ��
��ת����Һǰ����ƿ������������ˮ
�ݶ���ʱ����������ƿ�Ŀ̶���
���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��ߣ�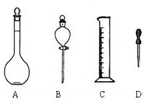
��1����ͼ��ʾ����������������Һ�϶������õ�����______������ĸ��������������Һ����Ҫ�IJ���������______�����������ƣ���
��2�����Ƹ���ҺӦѡ��______ mL����ƿ��ʹ������ƿ֮ǰ�������______��
��3�����Ƹ���ҺӦ��������ƽ��ȡ______ g������
��4��ʹ������ƿ������Һʱ�����ڲ��������������������������ʹ������ҺŨ��ƫ�͵���______�����ţ���
������ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ���
��������ƿ��ת����Һʱ������Һ����������ƿ����
����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ��
��ת����Һǰ����ƿ������������ˮ
�ݶ���ʱ����������ƿ�Ŀ̶���
���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��ߣ�

��1������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Բ���Ҫ�������з�Һ©�����ʴ�Ϊ��B���ձ�����������
��2����������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ������ƿ��ʹ��ǰҪ���м�©���ʴ�Ϊ��500ml����ƿ����©��
��3��������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ��CuSO4�����ʵ���n=cV=0.5L��0.1mol?L-1=0.05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0.05mol��250g/mol=12.5g���ʴ�Ϊ��12.5��
��4��������ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ��ˣ����������ƫС��Ũ��ƫС���ʢ���ȷ��
��������ƿ��ת����Һʱ������Һ����������ƿ���棬��Һ���к������ʣ����ʵ��������٣�Ũ��ƫС���ʢ���ȷ��
����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ�ӣ����ձ��Ͳ�������մ�����ʣ����ʵ��������٣�Ũ��ƫС���ʢ���ȷ��
��ת����Һǰ����ƿ������������ˮ������Һ�������Ӱ�죬Ũ�Ȳ��䣬�ʢܴ���
�ݶ���ʱ����������ƿ�Ŀ̶��ߣ���Һ��Һ��ȿ̶��߸ߣ���Һ�����ƫ��Ũ��ƫС���ʢ���ȷ��
���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��ߣ�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ��Ũ��ƫС���ʢ���ȷ��
��ѡ���٢ڢۢݢޣ�
��2����������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ������ƿ��ʹ��ǰҪ���м�©���ʴ�Ϊ��500ml����ƿ����©��
��3��������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ��CuSO4�����ʵ���n=cV=0.5L��0.1mol?L-1=0.05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0.05mol��250g/mol=12.5g���ʴ�Ϊ��12.5��
��4��������ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ��ˣ����������ƫС��Ũ��ƫС���ʢ���ȷ��
��������ƿ��ת����Һʱ������Һ����������ƿ���棬��Һ���к������ʣ����ʵ��������٣�Ũ��ƫС���ʢ���ȷ��
����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ�ӣ����ձ��Ͳ�������մ�����ʣ����ʵ��������٣�Ũ��ƫС���ʢ���ȷ��
��ת����Һǰ����ƿ������������ˮ������Һ�������Ӱ�죬Ũ�Ȳ��䣬�ʢܴ���
�ݶ���ʱ����������ƿ�Ŀ̶��ߣ���Һ��Һ��ȿ̶��߸ߣ���Һ�����ƫ��Ũ��ƫС���ʢ���ȷ��
���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��ߣ�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ��Ũ��ƫС���ʢ���ȷ��
��ѡ���٢ڢۢݢޣ�

��ϰ��ϵ�д�
�����Ŀ
 ���õ�����CuSO4?5H2O������480mL 0.1mol?L-1��CuSO4��Һ������������գ�
���õ�����CuSO4?5H2O������480mL 0.1mol?L-1��CuSO4��Һ������������գ�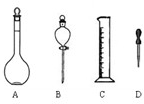 ���õ�����CuSO4?5H2O������480mL 0.1mol?L-1��CuSO4��Һ������������գ�
���õ�����CuSO4?5H2O������480mL 0.1mol?L-1��CuSO4��Һ������������գ�
